Abstract
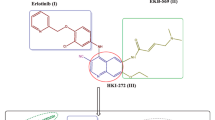
A library of 1,2,4-oxadiazole functionalized quinoline derivatives (13a–j) were synthesized and their structures were confirmed by 1H NMR, 13CNMR and Mass Spectral analysis. Further, these compounds were evaluated for their anticancer activity against four human cancer cell lines, namely MCF-7 (breast), A549 (lung), DU-145 (prostate) and MDA MB-231 (breast) using Etoposide as the positive control. Most of these derivatives exhibited more potent activity towards the four cancer cell lines compared to Etoposide. Amongst all the compounds tested, compounds 13b, 13c, 13h, 13i and 13j exhibited promising activity. Further of these compounds 13b, 13i and 13j exhibited excellent activity, when compared with Etoposide.


Similar content being viewed by others
References
Abadi AH, Hegazy GH, Zaher AAE (2005) Synthesis of novel 4-substituted-7- trifluoromethylquinoline derivatives with notric oxide releasing properties and their evaluation as analgesic and anti-inflammatory agents. Bioorg Med Chem 13:5759–5765
Agarwal M, Singh V, Sharma SC, Sharma P, Ansari MdY, Jadav SS, Yasmin S, Sreenivasulu R, Hassan MdZ, Saini P, Ahsan MJ (2016) Design and synthesis of new 2,5-disubstituted-1,3,4-oxadiazole analogues as anticancer agents. Med Chem Res 25:2289–2303
Ahsan MJ, Choudhary K, Jadav SS, Yasmin S, Ansari MY, Sreenivasulu R (2015) Synthesis, antiproliferative activity and molecular docking studies of curcumin, analogues bearing pyrazole ring. Med Chem Res 24:4166–4180
Antunes R, Batista H, Srivastava RM, Thomas G, Araujo CC, Longo RL, Magalhaes H, Leao MBC, Pava AC (2003) Synthesis, characterization and interaction mechanism of new ozadiazolo-pthalimides as peripheral analgesics. IV. J Mol Struct 660:1–13
Athri P, Wilson WD (2009) Molecular dynamics of water-mediated interactions of a linear benzimidazole-biphenyl diamidine with the DNA minor groove. J Am Chem Soc 131:7618–7625
Axel K, Jürgen E (2001) Pharmaceutical substances: syntheses, patents, applications. Thieme Chemistry, Stuttgart, Germany
Benard C, Zouhiri F, Normand-Bayle M, Danet M, Desmaele D, Leh H, Mouscadet JF, Mbemba G, Thomas CM, Bonnenfant S, Le Bret M, d’Angelo (2004) Linker-modified quinoline derivatives targeting HIV-1integrase: synthesis and biological activity. Bioorg Med Chem Lett 14:2473–2476
Bokach NA, Khripoun AV, Kukushkin VY, Haukka M, Pombeiro AJL (2003) A route to 1,2,3-Oxadiazoles and their complexes via platinum -mediated 1,3-dipolar cycloaddition of notrile oxides to organonitriles. Inorg Chem 42:896–903
Boys M, Schretzman L, Chandrakumar N, Tollefson M, Mohler S, Downs V, Penning T, Russell M, Wendt J, Chen B, Stenmark H, Wu H, Spangler D, Clare M, Desai B, Khanna I, Nguyen M, Duffin T, Engleman V, Finn M, Freeman S, Hanneke M, Keene J, Klover J, Nickols G, Nickols M, Steininger C, Westlin M, Westlin W, Yu Y, Wang Y, Dalton C, Norring S (2006) Convergent, parallel synthesis of a series of β- substituted 1,2,4-oxadiazole butanoic acids as potent and selectie αvβ3 recetor antagonists. Bioorg Med Chem Lett 16:839–844
Clitherow JW, Beswick P, Irving WJ, Scopes DIC, Barnes JC, Clapham J, Brown JD, Evans DJ, Hayes AG (1996) Novel 1,2,4-oxadiazoles as potent and selective histamine H3 receptor antagonists. Bioorg Med Chem Lett 6:833–838
Cottrell DM, Capers J, Salem MM, DeLuca-Fradley K, Croft SL, Werbovetz KA (2004) Antikinetoplastid activity of 3-aryl-5-thiocyanatomethyl-1,2,4-oxadiazoles. Bioorg Med Chem 12:2815–2824
De Martino G, La Regina G, Coluccia A, Edler MC, Barbera MC, Brancale A, Wilcox E, Hamel E, Artico M, Silvestri R (2004) Arylthioindoles, potent inhibitors of tubulin polymerization. J Med Chem 47:6120–6123
dos Santos Filho JM, Leite ACL, de Oliveira BG, Moreira DRM, Lima MS, Soares MBP, Leite LFCC (2009) Design, synthesis and cruzin docking of 3-(4-substituted-aryl)- 1,2,4-oxadiazole-N-acylhydrazones as anti-Trypanosoma cruzi agents. Bioorg Med Chem 17:6682–6691
Durgesh R, Sreenivasulu R, Srinivasarao P, Raju RR (2018a) Synthesis and anti-tumor evaluation of novel 5-bromo indole-aryl ketohydrazide-hydrazone analogues. Asian J Chem 30:1201–1204
Durgesh R, Sreenivasulu R, Srinivasarao P, Raju RR (2018b) Synthesis and anticancer evaluation of indazole-aryl hydrazide-hydrazone derivatives. J Ind Chem Soc 95:433–438
Durgesh R, Sreenivasulu R, Raju RR (2018c) Synthesis and anti-tumor evaluation of Indole-substituted Indole fused keto hydrazide-hydrazones. J Pharm Res 12:42–46
Eckhardt S (2002) Recent progress in the development of anticancer agents. Curr Med Chem 2:419–439
George Rosenker KM, Paquette WD, Johnston PA, Sharlow ER, Andreas V, Bakan A, Lazo JS, Wipf P (2015) Synthesis and biological wvaluation of 3-amino isoquinolin-1(2H)- one based inhibitors of the dual-specificity phosphatase Cdc25B. Bioorg Med Chem 23:2810–2818
Gupta SK, Mishra A (2016) Synthesis, characterization & screening for anti-inflammatory & analgesic activity of quinolone derivatives bearing azetidinones scaffolds. Antiinflamm Antiallergy Agents Med Chem 15:31–43
Hatti I, Sreenivasulu R, Jadav SS, Ahsan MJ, Raju RR (2015a) Synthesis and biological evaluation of 1,3,4-oxadiazole linked bis indole derivatives as anticancer agents. Monatsh Chem 146:1699–1705
Hatti I, Sreenivasulu R, Jadav SS, Jayaprakash V, Kumar CG, Raju RR (2015b) Synthesis, cytotoxic activity and docking studies of new 4-aza podophyllotoxin derivatives. Med Chem Res 24:3305–3313
Haugwitz RD, Martinez AJ, Venslavsky J, Angel RG, Maurer BV, Jacobs GA, Narayanan VL, Cruthers LR, Szanto J (1985) Antiparasitic agents. 6. Synthesis and anthelmintic activities of novel isothiocyanatophenyl-a,2,4-oxadiazoles. J Med Chem 2:1234–1241
Hemming K, Alan RK, Christopher AR, Eric FVS, Richard JKT (2008) Comprehensive Heterocyclic Chemistry III. Elsevier, Oxford, pp 243–314
Ispikoudi M, Amvrazis M, Kontogiorgis C, Koumbis AE, Litinas KE, Hadjipavlou-Litina D, Fylaktakidou KC (2010) Convenient synthesis and biological profile of 5-amino- substituted 1,2,4-oxadiazole derivatives. Eur J Med Chem 45:5635–5645
Jeschke P, Wachendorff-Neumann U, Erdelen C, Mencke N, Turberg A (1995) Ger. Offen. DE 4; 401,107 (Cl.C07D271/06), 20 Jul 1995, Appl. 17 Jan 1994, 40pp. Chem Abstr 123:340135t
Joshi AA, Narkhede SS, Viswanathan CL (2005) Design, synthesis and evaluation of 5- substituted amino-2,4-diamino-8-chloropyrimido-[4,5-b]quinolones as novel antimalarials. Bioorg Med Chem Lett 15:73–76
Jordan MA, Wilson L (1998) Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr Opin Cell Biol 10:123–130
Kiss LE, Ferreira HS, Torrao L, Bonifacio MJ, Palma PN, Soares-da-Silva P, Learmonth DA (2010) Discovery of a long-acting, preipherally selective inhibitor of catechol-O- methyltransferase. J Med Chem 53:3396–3411
Kumar A, Paliwal D, Saini D, Thakur A, Aggarwal S, Kaushik D (2014) A comprehensive review on synthetic approach for antimalarial agents. Eur J Med Chem 85:147–178
Lee CW, Hong DH, Han SB, Jong SH, Kim HC, Fine RL, Lee SH, Kim HM (2002) A novel stereo-selective sulfonylurea, 1-[1-(4-aminobenzoyl)-2,3-dihydro-1H-indol-6-sulfonyl]- 4-phenyl-imidazolidin-2-one, has antitumor efficacy in in vitro and in vivo tumor models. Biochem Pharmacol 64:473–480
Lueg C, Schepmann D, Günther R, Brust P, Wünsch B (2013) Development of fluorinated CB2 receptor agonists for PET studies. Bioorg Med Chem 21:7481–7498
Lu Y, Chen J, Xiao M, Li W, Miller DD (2012) An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm Res 29:2943–2971
Madhavi S, Sreenivasulu R, Ansari MdY, Ahsan MJ, Raju RR (2016) Synthesis, biological evaluation and molecular docking studies of Pyridine incorporated chalcone derivatives as anticancer agents. Lett Org Chem 13:682–692
Madhavi S, Sreenivasulu R, Jyotsna Y, Raju RR (2017a) Synthesis of Chalcone incorporated Quinazoline derivatives as anticancer agents. Saudi Pharm J 25:275–279
Madhavi S, Sreenivasulu R, Raju RR (2017b) Synthesis and biological evaluation of oxadiazole incorporated ellipticine derivatives as anticancer agents. Monatsh Chem 148:933–938
Maguire MP, Sheets KR, McVety K, Spada AP, Zilberstein A (1994) A new series of PDGF receptor tyrosine kinase inhibitors: 3-substituted quinoline derivatives. J Med Chem 37:2129–2137
Manfredini S, Lampronti I, Vertuani S, Solaroli N, Recanatini M, Bryan D, McKinney M (2000) Design, synthesis and binding at cloned muscarinic receptors of N-[5-(1’-substituted-acetoxymethyl)-3-oxadiazolyl] and N-[4-(1’-substituted-acteoxy methyl)-2-dioxolanyl] dialkyl amines. Bioorg Med Chem 8:1559–1566
Matsuki M, Hoshi T, Yamamoto Y, Ikemori‐Kawada M, Minoshima Y, Funahashi Y, Matsui J (2018) Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signalling pathways in human hepatocellular carcinoma models. Cancer Med 7:2641–2653
Najafi Z, Saeedi M, Mahdavi M, Sabourian R, Khanavi M, Tehrani MB, Moghadam FH, Edraki N, Karimpor-Razkenari E, Sharifzadeh M, Foroumadi A, Shafiee A, Akbarzadeh T (2016) Design and synthesis of novel anti-Alzheimer’s agents: acridine-chromenone and quinoline-chromenone hybrids. Bioorg Chem 67:84–94
Plattner J, Desai MC (2006) Comprehensive medicinal chemistry II. Elsevier, Amsterdam, London
Pragathi YJ, Sreenivasulu R, Veronica D, Madhavi S, Raju RR (2019) Design, synthesis and biological evaluation of novel 2-(4-arylsubstituted-1H-1,2,3-triazol-1-yl)-N-(4-(2- (thiazol-2-yl)benzo[d]thiazol-6-yl)phenyl) acetamide derivatives as potent anticancer agents. Russian J Gen Chem 89:1009–1014
Reddy NB, Burra VR, Ravindranath LK, Sreenivasulu R, Kumar VN (2016a) Synthesis and biological evaluation of benzoxazole fused combretastatin derivatives as anticancer agents. Monatsh Chem 147:593–598
Reddy NB, Burra VR, Ravindranath LK, Kumar VN, Sreenivasulu R, Sadanandam P (2016b) Synthesis and biological evaluation of benzimidazole fused ellipticine derivatives as anticancer agents. Monatsh Chem 147:599–604
Roma G, Di Braccio M, Grossi G, Mattioli F, Ghia M (2000) 1,8-Naphthyridines IV. 9- substituted N,N-dialkyl-5-(alkylamino or cycloalkylamino)[1,2,4]triazolo[4,3- a][1,8]naphtha yridine-6-carboxamides, new compounds with anti-aggressive and potent anti-inflammatory activities. Eur J Med Chem 35:1021–1035
Shahinshavali SK, Sreenivasulu R, Guttikonda VR, Kolli D, Rao MVB (2019) Synthesis and biological evaluation of amide derivatives of 1,2-isoxazole fused 1,2,4-thiadiazole as anticancer agents. Russian J Gen Chem 89:324–329
Sreenivasulu R, Sujitha P, Jadav SS, Ahsan MJ, Kumar CG, Raju RR (2017) Synthesis, antitumor evaluation and molecular docking studies of Indole–Indazolyl hydrazide– hydrazone derivatives. Monatsh Chem 148:305–314
Sreenivasulu R, Durgesh R, Jadav SS, Sujitha P, Kumar CG, Raju RR (2018) Synthesis, anticancer evaluation and molecular docking studies of bis(indolyl)triazinones, Nortopsentin analogs. Chem Pap 72:1369–1378
Sreenivasulu R, Reddy KT, Jadav SS, Sujitha P, Kumar CG, Raju RR (2019) Synthesis, antiproliferative and apoptosis induction potential activities of novel bis(indolyl)hydrazide-hydrazone derivatives. Bioorg Med Chem 27:1043–1055
Solomon VR, Lee H (2011) Quinoline as a privileged scaffold in cancer drug iscovery. Curr Med Chem 18:1488–1508
Spandana Z, Sreenivasulu R, Rao MVB (2019a) Design, synthesis and anticancer evaluation of carbazole fused aminopyrimidine derivatives. Lett Org Chem 16:662–667
Spandana Z, Sreenivasulu R, Rekha TM, Rao MVB (2019b) Novel 1,3,4-oxadiazole fused thiadiazole derivatives: synthesis and study of anticancer activities. Lett Drug Des Disco 16:656–662
Subramanyam M, Sreenivasulu R, Rambabu G, Rao MVB, Rao KP (2018) Synthesis, biological evaluation and docking studies of 1,3,4-oxadiazole fused benzothiazole derivatives for anticancer drugs. Lett Drug Des Disco 15:1299–1307
Suma VR, Sreenivasulu R, Subramanyam M, Rao KRM (2019) Design, synthesis and anticancer evaluation of amide derivatives of structurally modified Combretastatin A4 as anticancer agents. Russian J Gen Chem 89:499–504
Wen LR, Sun QC, Zhang HL, Li M (2013) A new rapid multicomponent domino heteroannulation of heterocyclic ketene aminals: solvent-free regioselective sythesis of functionalized benzo[g]imidazo[1,2-a]quinolinediones. Org Biomol Chem 11:781–786
Xu J, Wei L, Mathvink R, He J, Park YJ, He H, Leiting B, Lyons KA, Marsilio F, Patel RA, Wu JK, Thornberry NA, Weber AE (2005) Discovery of potent and selective phenylalanine based dipeptidyl peptidase IV inhibitors. Bioorg Med Chem Lett 15:2533–2536
Yakantham T, Sreenivasulu R, Raju RR (2019) Design, synthesis and anticancer evaluation of 2-(3-(4-((5-aryl-1,2,4-oxadiazol-3-yl)methoxy)phenyl)isoxazol-5-yl)-N-(3,4,5-trimeth yl phenyl) thiazol-4-amine derivatives. Russ J Gen Chem 89:1485–1490
Zajdel P, Partyka A, Marciniec K, Bojarski AJ, Pawlowski M, Wesolowska A (2014) Quinoline- and isoquinoline-sulfonamide analogs of aripiprazole: novel antipsychotic agents? Future Med Chem 6:57–75
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kala, P., Khasim Sharif, S., Murali Krishna, C. et al. Design, synthesis, and anticancer evaluation of 1,2,4-oxadiazole functionalized quinoline derivatives. Med Chem Res 29, 136–144 (2020). https://doi.org/10.1007/s00044-019-02467-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02467-6