Abstract
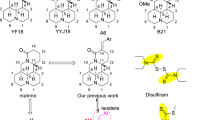
Using matrine (1) as the lead compound, a series of new piperazinyl matrine derivatives were designed, synthesized and evaluated for their antitumor activities in vitro and in vivo. Structure activity relationship (SAR) analysis indicated that introduction of substituted piperazine on matrine might significantly enhance the antiproliferative activity. Moreover, types of substituents of piperazine exhibited great different effects on the antiproliferative activity of target compounds against Bel-7402 and RKO cell lines. The in vivo antitumor assay results revealed that some of the target derivatives possessed better therapeutic efficacy than matrine and low toxicity. More importantly, among the newly synthesized compounds, M16 and M26 possessed strong antitumor activity against the two cell lines. Moreover, six of the synthesized compounds M1, M3, M7, M10, M11 and M17 proved to be of much better therapeutic efficacy than matrine via in vivo antitumor assay. The study provides a theoretical basis for further structural optimizations and discovery of the antitumor pathways of this kind of compounds.




Similar content being viewed by others
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
References
Aghvami M, Ebrahimi F, Zarei MH et al. (2018) Matrine induction of ROS mediated apoptosis in human ALL B-lymphocytes Via mitochondrial targeting. Asian Pac J Cancer Prev 19:555–560
Dai Z-J, Gao J, Ji Z-Z, Wang X-J, Ren H-T, Liu X-X, Wu W-Y, Kang H-F, Guan H-T (2009) Matrine induces apoptosis in gastric carcinoma cells via alteration of Fas/FasL and activation of caspase-3 J Ethnopharmacol 123:91–96
Fajas L, Benfodda Z, Fritz V (2011) Preparation of piperazine derivatives as inhibitors of stearoyl-CoA-desaturase-1enzyme. WO 2011/030312 A1. 2011.
Hamdy AA (2012) Cancer therapy and vaccination. J Immunol Methods 382:1–23
Jones P, Maria Ontoria OJ (2011) Preparation of piperidine and piperazine derivatives as SMO antagonist. WO 2011/036478 A1. 2011.
Kimura M, Masuda T, Yamada K, Kawakatsu N, Kubota N, Mitani M, Kishii K, Inazu M, Kiuchi Y, Oguchi K, Namiki T (2004) Antioxidative activities of novel diphenylalkyl piperazine derivatives with high affinities for the dopamine transporter. Bioorg Med Chem Lett 14:4287–4290
Lacivita E, Leopoldo M, De Giorgio P, Berardi F, Perrone R (2009) Determination of 1-aryl-4-propylpiperazine pKa values: the substituent on aryl modulates basicity. Bioorg Med Chem 17:1339–1344
Lin Y, Lin L, Jin Y, Wang D, Tan Y, Zheng C (2014) Combination of matrine and sorafenib decreases the aggressive phenotypes of hepatocellular carcinoma cells. Chemotherapy 60:112–118
Rathi AK, Syed R, Shin H-S, Patel RV (2016) Piperazine derivatives for therapeutic use: a patent review (2010-present). Expert Opin Ther Pat 26:777–797
Schiemann K, Schultz M, Staehle W (2010) Piperidine and piperazine derivatives as autotaxin inhibitors and their preparation and use for the treatment and prophylaxis of cancers and other autotaxin-mediated diseases. WO 2010115491 A2. 2010.
Shapiro LA, Offord SJ, Ordway GA (1995) The effect of chronic treatment with a novel aryl-piperazine antipsychotic on monoamine receptors in rat brain. Brain Res 677:250–260
Solomon VR, Hu C, Lee H (2010) Design and synthesis of anti-breast cancer agents from 4-piperazinylquinoline: a hybrid pharmacophore approach. Bioorg Med Chem 18:1563–1572
Yang C, Xu G, Li J, Wu X, Liu B, Yan X, Wang M, Xie Y (2005) Benzothiophenes containing a piperazine side chain as selective ligands for the estrogen receptor α and their bioactivities in vivo. Bioorg Med Chem Lett 15:1505–1507
Zhang GL, Jiang L, Yan Q, Liu RH, Zhang L (2015) Anti-tumor effect of matrine combined with cisplatin on rat models of cervical cancer. Asian Pac J Trop Med 8:1055–1059
Zhang JQ, Li YM, Liu T et al. (2010) Antitumor effect of matrine in human hepatoma G2 cells by inducing apoptosis and autophagy. World J Gastroenterol 16:4281–4290
Acknowledgements
We are grateful to Prof. Chen Rui, Faculty of Chinese Medicine Science, Guangxi University of Chinese Medicine for proofreading of the manuscript.
Author contributions
Y.X. and P.L. performed research and drafted the initial manuscript. H.R. revised and approved the manuscript. L.W. helped in biological activity. P.X modified the language. H.W. and S.Z. contributed the compounds synthesis. L.W. and J.J. designed the research.
Funding
This work was supported by the National Natural Science Foundation of China (21403225), the Innovation Project of Guangxi Graduate Education (YCBZ2019026), the high-level innovation team and outstanding scholar project of Guangxi institutions of higher education (guijiaoren (2014) 49 hao), Guangxi Natural Science Foundation (2017GXNSFBA198240) and the State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, Guangxi Normal University (CMEMR2017-B14).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All protocols and care of the mice were performed in strict compliance with Guidelines for the Use of Laboratory Animals (National Research Council) and approved by the SPF Animal Laboratory of Guangxi University.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Xu, Y., Liang, P., Rashid, H.u. et al. Design, synthesis, and biological evaluation of matrine derivatives possessing piperazine moiety as antitumor agents. Med Chem Res 28, 1618–1627 (2019). https://doi.org/10.1007/s00044-019-02398-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02398-2