Abstract
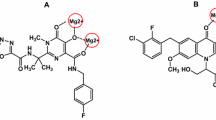
A new class of 2-benzoxazolinone derivatives was designed and synthesized for its anti-human immunodeficiency virus-1 activity. The benzoxazolinone scaffold could be replaced with catechol moiety in the potent but toxic integrase strand transfer inhibitors. The biological evaluation of the synthesized compounds revealed that all compounds were active against human immunodeficiency virus-1 at 100 μM. It is also found that most of the compounds presented no significant cytotoxicity at concentration of 100 μM. The most potent compound with thiadiazole ring as the linker inhibited the human immunodeficiency virus-1 with 84% rate. Docking of this structure in the active site of prototype foamy virus integrase indicated that the chelation of two Mg2+ cations might be the probable mechanism of the anti-human immunodeficiency virus-1 activity. Our results indicated that the synthesized compounds can provide a very good basis for the development of new anti-human immunodeficiency virus-1 agents.





Similar content being viewed by others
References
Al-Mawsawi LQ, Dayam R, Taheri L, Witvrouw M, Debyser Z, Neamati N (2007) Discovery of novel non-cytotoxic salicylhydrazide containing HIV-1 integrase inhibitors. Bioorg Med Chem Lett 17:6472–6475
Bach A, Pizzirani D, Realini N, Vozella V, Russo D, Penna I, Melzig L, Scarpelli R, Piomelli D (2015) Benzoxazolone carboxamides as potent acid ceramidase inhibitors: synthesis and structure–activity relationship (SAR) studies. J Med Chem 58:9258–9272. doi:10.1021/acs.jmedchem.5b01188
Billamboz M, Bailly F, Lion C, Calmels C, Andréola M-L, Witvrouw M, Christ F, Debyser Z, De Luca L, Chimirri A, Cotelle P (2011) 2-Hydroxyisoquinoline-1,3(2H,4H)-diones as inhibitors of HIV-1 integrase and reverse transcriptase RNase H domain: Influence of the alkylation of position 4. Eur J Med Chem 46:535–546. doi:10.1016/j.ejmech.2010.11.033
Boros EE, Johns BA, Garvey EP, Koble CS, Miller WH (2006) Synthesis and HIV-integrase strand transfer inhibition activity of 7-hydroxy [1, 3] thiazolo [5, 4-b] pyridin-5 (4H)-ones. Bioorg Med Chem Lett 16:5668–5672
Chi G, Nair V, Semenova E, Pommier Y (2007) A novel diketo phosphonic acid that exhibits specific, strand-transfer inhibition of HIV integrase and anti-HIV activity. Bioorg Med Chem Lett 17:1266–1269
Dayam R, Al-Mawsawi LQ, Neamati N (2007) Substituted 2-pyrrolinone inhibitors of HIV-1 integrase. Bioorg Med Chem Lett 17:6155–6159
De Clercq E, Poupaert JH (1999) Synthesis and antiviral activity of 6-benzoyl-benzoxazolin-2-one and 6-benzoyl-benzothiazolin-2-one derivatives. Antivir Chem Chemother 10:87–97
Gerova MS, Stateva SR, Radonova EM, Kalenderska RB, Rusew RI, Nikolova RP, Chanev CD, Shivachev BL, Apostolova MD, Petrov OI (2016) Combretastatin A-4 analogues with benzoxazolone scaffold: Synthesis, structure and biological activity. Eur J Med Chem 120:121–133. doi:10.1016/j.ejmech.2016.05.012
Hajimahdi Z, Zarghi A, Zabihollahi R, Aghasadeghi M (2013) Synthesis, biological evaluation, and molecular modeling studies of new 1, 3, 4-oxadiazole-and 1, 3, 4-thiadiazole-substituted 4-oxo-4H-pyrido [1, 2-a] pyrimidines as anti-HIV-1 agents. Med Chem Res 22:2467–2475
Hassounah SA, Mesplède T, Wainberg MA (2016) Nonhuman primates and humanized mice for studies of HIV-1 integrase inhibitors: a review. Pathogens Immunity 1:41–67
Ingale KB, Bhatia MS (2011) HIV-1 integrase inhibitors: a review of their chemical development. Antivir Chem Chemother 22:95–105
Koeksal M, Goekhan N, Kuepeli E, Yesilada E, Erdoğan H (2005) Synthesis, Analgesic and Antiinflammatory properties of certain 5‐/6‐Acyl‐3‐(4‐substituted‐1‐piperazinylmethyl)‐2‐benzoxazolinones Derivatives. Arch Pharm (Weinheim) 338:117–125
Li B-W, Zhang F-H, Serrao E, Chen H, Sanchez TW, Yang L-M, Neamati N, Zheng Y-T, Wang H, Long Y-Q (2014) Design and discovery of flavonoid-based HIV-1 integrase inhibitors targeting both the active site and the interaction with LEDGF/p75. Bioorg Med Chem 22:3146–3158
Lin C-C, Cheng H-Y, Yang C-M, Lin T-C (2002) Antioxidant and antiviral activities ofEuphorbia thymifolia L. J Biomed Sci 9:656–664
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662
Pawar R, Das T, Mishra S, Pancholi B, Gupta SK, Bhat SV (2014) Synthesis, anti-HIV activity, integrase enzyme inhibition and molecular modeling of catechol, hydroquinone and quinol labdane analogs. Bioorg Med Chem Lett 24:302–307
Poupaert J, Carato P, Colacino E (2005) 2 (3H)-benzoxazolone and bioisosters as “privileged scaffold” in the design of pharmacological probes. Curr Med Chem 12:877–885
Quashie PK, Sloan RD, Wainberg MA (2012) Novel therapeutic strategies targeting HIV integrase. BMC Med 10:1
Salgın-Gökşen U, Gökhan-Kelekçi N, Göktaş Ö, Köysal Y, Kılıç E, Işık Ş, Aktay G, Özalp M (2007) 1-Acylthiosemicarbazides, 1, 2, 4-triazole-5 (4H)-thiones, 1, 3, 4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg Med Chem 15:5738–5751
Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR (1988) Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res 48:4827–4833
Stranix BR, Wu JJ, Milot G, Beaulieu F, Bouchard JE, Gouveia K, Forte A, Garde S, Wang Z, Mouscadet JF, Delelis O, Xiao Y (2016) Pyridoxine hydroxamic acids as novel HIV-integrase inhibitors. Bioorg Med Chem Lett 26:1233–1236. doi:10.1016/j.bmcl.2016.01.028
Tanis SP, Plewe MB, Johnson TW, Butler SL, Dalvie D, DeLisle D, Dress KR, Hu Q, Huang B, Kuehler JE (2010) Azaindole N-methyl hydroxamic acids as HIV-1 integrase inhibitors-II. The impact of physicochemical properties on ADME and PK. Bioorg Med Chem Lett 20:7429–7434
Wang Z, Vince R (2008) Synthesis of pyrimidine and quinolone conjugates as a scaffold for dual inhibitors of HIV reverse transcriptase and integrase. Bioorg Med Chem Lett 18:1293–1296
Yu S, Sanchez TW, Liu Y, Yin Y, Neamati N, Zhao G (2013) Design and synthesis of novel pyrimidone analogues as HIV-1 integrase inhibitors. Bioorg Med Chem Lett 23:6134–6137. doi:10.1016/j.bmcl.2013.09.018
Zabihollahi R, Sadat S, Vahabpour R, Aghasadeghi M, Memarnejadian A, Ghazanfari T, Salehi M, Rezaei A, Azadmanesh K (2010) Development of single-cycle replicable human immunodeficiency virus 1 mutants. Acta Virol 55:15–22. doi:10.4149/av_2011_01_15
Acknowledgements
This study was financially supported by Research Deputy of Shahid Beheshti University of Medical sciences as part of Ph. D thesis of M. Safakish.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Safakish, M., Hajimahdi, Z., Zabihollahi, R. et al. Design, synthesis, and docking studies of new 2-benzoxazolinone derivatives as anti-HIV-1 agents. Med Chem Res 26, 2718–2726 (2017). https://doi.org/10.1007/s00044-017-1969-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1969-8