Abstract
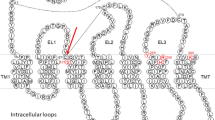
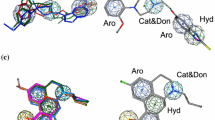
P2Y12 receptor becomes a favorable target for therapeutic antiplatelet agents. Reversible P2Y12 receptor antagonists have more benefits than the irreversible ones on rapid absorption, onset of action and higher inhibition of platelet aggregation. As potent reversible P2Y12 receptor antagonists, piperazinyl-glutamate-pyridines exhibit a significant improvement in efficacy while maintaining a better safety profile than the irreversible ones. In this study, we developed a 3D pharmacophore model with r 2 = 0.966 based on a set of piperazinyl-glutamate-pyridines showing the important pharmacophore features (one hydrogen bond acceptor, one hydrogen bond donor, one ring aromatic and one hydrophobic feature) which is necessary for reversible P2Y12 receptor antagonists. The obtained pharmacophore models were validated by using well-known methodologies such as test molecules and Fischer’s randomization method. A docking study was also performed based on the recently released P2Y12 receptor crystal structure (PDB ID: 4NTJ) which revealed key residues (Tyr105, His 187 and Lys280) in the receptor–ligand interaction. The docking results were well in agreement with the pharmacophore model that raised the model’s reliability. The pharmacophore model was further confirmed by estimating the activity of six known drugs which can distinguish the reversible P2Y12 receptor antagonists from the irreversible ones. The results of our study provide confidence for the utility of the selected chemical features to retrieve further compounds as reversible P2Y12 receptor antagonists.







Similar content being viewed by others
References
Accelrys Software Inc. (2005) Discovery studio modeling environment, Accelrys Software Inc., San Diego
Aillaud I et al (2010) 2-chlorophenyl zinc bromide: a convenient nucleophile for the mannich-related multicomponent synthesis of clopidogrel and ticlopidine. Molecules 15(11):8144–8155
Akinosoglou K, Alexopoulos D (2014) Use of antiplatelet agents in sepsis: a glimpse into the future. Thromb Res 133(2):131–138
Angiolillo DJ, Ferreiro JL (2010) Platelet adenosine diphosphate P2Y12 receptor antagonism: benefits and limitations of current treatment strategies and future directions. Rev Esp Cardiol 63(1):60–76
Aslama M, Sedding D, Koshty A, Santosoc S, Schulzd R, Hamma C, Gündüz D (2013) Nucleoside triphosphates inhibit ADP, collagen, and epinephrine-induced platelet aggregation: role of P2Y1 and P2Y12 receptors. Thromb Res 132(5):548–557
Barn K, Steinhubl SR (2012) A brief review of the past and future of platelet P2Y12 antagonist. Coron Artery Dis 23(6):368–374
Bernlochner I, Sibbing D (2012) Thienopyridines and other ADP-receptor antagonists. Handb Exp Pharmacol 210(210):165–198
Cattaneo M (2010) New P2Y12 Inhibitors. Circulation 121:171–179
Gurbel PA et al (2003) Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 107(23):2908–2913
Hao M et al (2011) Combined 3D-QSAR, molecular docking, and molecular dynamics study on piperazinyl-glutamate-pyridines/pyrimidines as potent P2Y12 antagonists for inhibition of platelet aggregation. J Chem Inf Model 51(10):2560–2572
Kubica J, Kozinski M, Navarese EP et al (2014) Cangrelor: an emerging therapeutic option for patients with coronary artery disease. Curr Med Res Opin 30(5):813–828
Kurogi Y, Guner OF (2001) Pharmacophore modeling and three-dimensional database searching for drug design using catalyst. Curr Med Chem 8(9):1035–1055
Li P, Guangyin Z, Yi D, Yang C, Junping Z (2011) Role of platelet-monocyte aggregation in the onset of acute cardiovascular and cerebrovascular diseases. Mode Tradit Chin Med Mater Med. 13(3):493–497
Lin K-J et al (2013) The structural characteristics of cannabinoid type 1 receptor antagonists. Chem J Chin Univ 34(5):1240–1245
Liu H, Ge H, Peng Y, Xiao P, Xu J (2011) Molecular mechanism of action for reversible P2Y12 antagonists. Biophys Chem 155:74–81
Meadows TA, Bhatt DL (2007) Clinical aspects of platelet inhibitors and thrombus formation. Circ Res 100(9):1261–1275
Mitra I, Saha A, Roy K (2010) Pharmacophore mapping of arylamino-substituted benzo[b]thiophenes as free radical scavengers. J Mol Model 16(10):1585–1596
Parlow JJ, Burney MW (2009) Piperazinyl-glutamate-pyridines as potent orally bioavailable P2Y12 antagonists for inhibition of platelet aggregation. Bioorg Med Chem Lett 19:4657–4663
Parlow JJ, Burney MW (2010) Part II: piperazinyl-glutamate-pyridines as potent orally bioavailable P2Y12 antagonists for inhibition of platelet aggregation. Bioorg Med Chem Lett 20:1388–1394
Parlow JJ, Mary WB, Case BL, Girard TJ, Hall KA, Harris PK, Hiebsch RR, Huff RM, Lachance RM, Mischke DA, Rapp SR, Woerndle RS, Ennis MD (2010) Piperazinyl glutamate pyridines as potent orally bioavailable P2Y12 antagonists for inhibition of platelet aggregation. J Med Chem 53(5):2010–2037
Sabouret P, Taiel-Sartral M (2014) New antiplatelet agents in the treatment of acute coronary syndromes. Arch Cardiovasc Dis 107(3):178–187
Sakkiah S, Baek A, Lee KW (2012) Pharmacophore modeling and molecular dynamics simulation to identify the critical chemical features against human sirtuin 2 inhibitors. J Mol Struct 1011:66–75
Serebruany VL (2012) Viewpoint: reversible nature of platelet binding causing transfusion-related acute lung injury (TRALI) syndrome may explain dyspnea after ticagrelor and elinogrel. Thromb Haemost 108(6):1024–1027
Springthorpe B, Briley A, Barton P (2007) From ATP to AZD6140: the discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorg Med Chem Lett 17(21):6013–6018
Zhang H et al (2012) Synthesis and biological evaluation of ticagrelor derivatives as novel antiplatelet agents. Bioorg Med Chem Lett 22(11):3598–3602
Zhang K et al (2014a) Structure of the human P2Y12 receptor in complex with an antithrombotic drug. Nature 509:115–118
Zhang J et al (2014b) Agonist-bound structure of the human P2Y12 receptor. Nature 509:119–122
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, Z., Wu, G., Wang, C. et al. Features of reversible P2Y12 receptor antagonists based on piperazinyl-glutamate-pyridines. Med Chem Res 25, 1204–1215 (2016). https://doi.org/10.1007/s00044-016-1557-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1557-3