Abstract
P2Y receptors are G-protein-coupled receptors (GPCRs) for extracellular nucleotides. There are eight mammalian P2Y receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14). The widely expressed P2Y receptors play important roles in physiology and pathophysiology. This review summarizes the use of pharmacological tools to characterize the P2Y receptor subtypes involved in these responses. MRS2500 is a potent and selective antagonist acting at the P2Y1 receptor. AR-C118925 is useful for the selective antagonism of the P2Y2 receptor. PSB16133 blocks the P2Y4 receptor, MRS2578 is an antagonist at the P2Y6 receptor and NF157 as well as NF340 block the P2Y11 receptor. ADP-induced platelet aggregation is mediated by P2Y1 and P2Y12 receptors. A number of compounds or their active metabolites reduce ADP-induced platelet aggregation by blocking the P2Y12 receptor. These include the active metabolites of the thienopyridine compounds clopidogrel and prasugrel, the nucleoside analogue ticagrelor and the nucleotide analogue cangrelor. PSB0739 is also a potent antagonist at the P2Y12 receptor useful for both in vitro and in vivo studies. MRS2211 and MRS2603 inhibit P2Y13 mediated responses. PPTN is a very potent antagonist at the P2Y14 receptor.
Similar content being viewed by others
This article is dedicated to late Professor María Teresa Miras-Portugal. She was a leading scientist in the field of purinergic signaling and P2 receptors for extracellular nucleotides [111,112,113,114]. She contributed to many important studies, advisory and editorial boards, the Purine club and the IUPHAR sub-committee for the nomenclature of P2Y receptors. She was the expert in the field of biochemistry, physiology and pharmacology of dinucleoside polyphosphates.
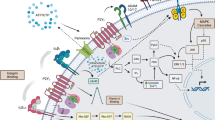
There are eight human subtypes of G-protein-coupled receptors (GPCRs) for extracellular nucleotides, P2Y receptors [23, 89]. They belong to the delta-subgroup of class A GPCRs [49]. The P2Y receptor family can be divided into two subfamilies [2, 156]. The first subfamily consists of the P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 receptors. The receptors couple via Gq-proteins to stimulation of phospholipase C (references in Tables 1, 2, 3, 4, and 5). P2Y11-receptors couple in addition through Gs and increases in adenylate cyclase activity (references in Table 5). In contrast, the P2Y12 receptor subfamily (P2Y12, P2Y13, and P2Y14 receptors) signals through activation of Gi-proteins (references in Tables 6, 7, and 8; see also [83, 158, 160]. P2Y receptors play important roles in physiology and pathophysiology [2, 22, 24, 120, 138, 139, 156, 158, 159]. Genetic knockout models can be used to identify the subtypes involved in responses to nucelotides. A fast alternative is the use of subtype specific ligands modifying cellular or tissue responses. Due to the efforts of medicinal chemistry novel compounds have been developed [10, 80,81,82, 118, 119, 121, 135, 143]. This article discusses pharmacological tools used to characterize P2Y receptor subtypes.
When using nucleotides such as ATP as agonists an interaction with ecto-nucleotidases [172] may be important. Hence, ATP may be degraded to ADP and further to adenosine which may activate adenosine receptors [74].
The P2Y1 receptor plays important roles in physiology including vasodilation, platelet aggregation, pain sensation, and astroglial signaling [2, 12, 48, 78, 124]. ADP is the principal agonist acting at the human P2Y1 receptor Table 1, [7, 84, 98]. Application of ATP may induce responses mediated by the P2Y1 receptor due to the fast breakdown of ATP to ADP [172]. When studied in detail with purified nucleotides, ATP has also been shown to act as a partial agonist at the receptor [64, 161]. 2-Methylthio-ADP activates the receptor (Table 1). The Northern (N-methanocarba analogue of 2-methylthio-ADP MRS2365; Table 1; [30] is a very potent and selective agonist that is inactive at the other two ADP receptors, P2Y12 and P2Y13.
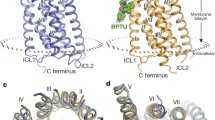
Bisphosphate analogues including MRS2279 (2-chloro-N6-methyl-(N)-methanocarba-2´-deoxyadenosine 3´,5´-bisphosphate; [162] and MRS2500 (2-iodo-N6-methyl-(N)-methanocarba-2´-deoxyadenosine 3´,5´-bisphosphate; [91] act as antagonists. MRS2500 is a potent and selective antagonist Table 1, [91]. 2,2´-Pyridylisatogen tosylate [54] and BPTU (N-[2-[2-(1,1-dimethylethylphenoxy]-3-pyridinyl]-N'-[4-(trifluoromethoxyphenyl]urea; [28, 171] act as allosteric inhibitors. Interestingly, MRS2500 and BPTU have been used to grow crystals for the crystallography of the human P2Y1 receptor protein [171]. The data of that study clearly show two disparate ligand-binding sites at the receptor protein [171]. MRS2500 binds within the seven transmembrane helixes and BPTU binds to an allosteric pocket on the receptor interface with the cell membrane [171].
([32P]-labeled and [125I]-labeled) analogues of MRS2500 can be used for binding studies [73, 124], Table 1. A [18F]PET tracer ([18F]1-{2-[2-(tert-butyl)phenoxy]pyridin-3-yl}-3-[4-(2-fluoroethyl)phenyl]urea) is available for imaging of the tissue distribution of P2Y1 receptors [116].
FormalPara P2Y2 receptor ligandsThe P2Y2 receptor plays roles in ion transport, regulation of epithelial cells, migration, vasodilatation and immune responses [31, 44, 97, 133, 140, 141]. Both ATP and UTP activate the receptor Table 2, [45]. Diadenosine-tetraphosphate (Ap4A), Up4U (diquafosol, INS365 [96, 131], and P1-(uridine 5´)-P4-(2´-deoxycytidine-5´)tetraphosphate (INS37217, denufosol; [83] also act as agonists. In fact, diquafosol is used in Japan and South Korea for the treatment of the dry eye syndrome [153, 165]. The 2-thio-analogues of UTP MRS2698 and PSB-1114 are more selective P2Y2 receptor agonists Table 2, [43, 77].
AR-C118925 is a potent and selective non-nucleotide antagonist at the P2Y2 receptor Table 2; [87, 92, 136, 137]. A fluorescent analogue of AR-C118925 can be used for labeling of the receptor at the cell membrane [37].
FormalPara P2Y4 receptor ligandsThe P2Y4 receptor controls cellular responses including ion transport, growth and migration [71, 142]. The human P2Y4 receptor is activated by UTP and blocked by ATP Table 3, [32, 88, 123]. In contrast, both UTP and ATP act as full agonists at the rat P2Y4 receptor [21]. CTP analogues including MRS4062, and N4-(phenylethoxy)-CTP activate the human P2Y4 receptor with a preference over P2Y2 and P2Y6 receptors Table 3, [108].
PSB-1699 and PSB-16133 inhibit P2Y4 receptor activation with a clear selectivity versus other P2Y receptor subtypes [137]. They may act as allosteric inhibitors [137].
FormalPara P2Y6 receptor ligandsThe human P2Y6 receptor plays roles vasoconstriction, ion secretion, migration, and inflammation [58, 60, 95, 97, 100, 103, 115, 134]. UDP is the endogenous agonist activating the receptor Table 4, [33, 123]. Synthetic agonists include PSB-0474 [43], MRS2693 [17] and the R(p) isomer of 5-OMe-UDP(α-B) Table 4, [59, 79].
MRS2578 blocks the P2Y6 receptor in a non-surmountable manner Table 4; [104]. MRS2578 may exert off target effects inhibiting cell migration [145]. TIM-38 (3-nitro-2-(trifluoromethyl)-2H-chromene) is a novel antagonist inhibiting P2Y6 receptor activation with an IC50 value of 4.3 μM and selectivity versus other P2Y receptor subtypes [76].
FormalPara P2Y11 receptor ligandsThe P2Y11 receptor plays roles in granulocyte differentiation, dendritic cell maturation, chemotactic responses of neutrophils and neuropathic pain [164]. The human P2Y11 receptor is activated by ATP Table 5, [34]. In contrast, ADP is the principal agonist activating the canine P2Y11 receptor [166]. Synthetic agonists include the P2Y12 receptor antagonist 2-propylthio-β,γ-dichloromethylene-D-ATP (AR-C67085) and the suramin analogue NF546 Table 5, [109].
Suramin and the suramin analogues NF157 and NF340 act as antagonists at the human P2Y11 receptor Table 5; [109, 154]. NF340 is selective and slightly more potent Table 5, [109].
FormalPara P2Y12 receptor ligandsThe P2Y12 receptor plays a crucial role in ADP-induced platelet aggregation [26, 63, 70, 152, 168]. Receptor activation is also involved in vasoconstriction, control of microglia, immune responses and control of bone mass [5, 16, 62, 72, 105, 115, 148, 151, 163]. As mentioned above, ADP is the endogenous agonist acting at the P2Y12 receptor Table 6, [30, 70]. ATP has antagonistic properties [86]. 2-Methylthio-ADP is a more potent agonist (Table 6).
Reduction of platelet aggregation is an important strategy in the prevention or therapy of cardiovascular events such as myocardial infarction. A number of potent antagonists have been developed (Table 6). AR-C67085 and cangrelor (AR-C69931MX; [75] are surmountable antagonists. Cangrelor is used to reduce platelet aggregation by intravenous application [99]. Cangrelor also blocks the P2Y13 receptor in a non-competitive manner see below Table 7, [106].
The radiolabeled nucleotide derivative [3H]PSB-0413 has a nanomolar affinity at the P2Y12 receptor Table 6; [42, 125]. The nucleoside analogue ticagrelor (AZD6140) is orally available and used for the reduction of platelet aggregation in patients after myocardial infarction [126, 155]. Ticagrelor appears to act in a surmountable manner at the human P2Y12 receptor Table 6, [68], see also [6, 57, 129]. In addition to the P2Y12 receptor, ticagrelor also blocks the P2Y13 receptor [19] and the equilibrative nucleoside transporter 1 [6]. Non-nucleotide antagonists include the analogue of reactive blue 2, PSB-0739 Table 6, [11, 67], 6-amino-2-thio-3H-pyrimidin-4-one derivatives [38], ethyl 6-aminonicotinate acyl sulfonamides [8], flavonolignans [18], morpholine derivatives [3], piperazinyl glutamates [130, 167], as well as salvianolic acids [101]. Selatogrel (ACT-246475) has a nanomolar affinity at the human P2Y12 receptor [9] and can be applied by subcutaneous administration [14, 147]. Selatogrel may act as inverse agonist at the human P2Y12 receptor [132].
Thienopyridine compounds are used for decades to reduce platelet aggregation in patients with cardiovascular diseases [15, 26]. The active metabolites of clopidogrel Table 6, [144], and prasugrel [149, 150] interact in an irreversible manner with Cys973.25 of the receptor protein (see red arrow in Fig. 1, [4, 41, 144]. Treatment with prasugrel is more potent when compared to ticlopidine or clopidogrel [15].
Predicted two-dimensional structure of the human P2Y12 receptor. TM transmembrane region, EL extracellular loop. Modified from [156]. The red arrow indicates Cys97 that is important for the interaction with the active metabolites of clopidogrel and prasugrel [4, 41, 144] and plays a role in receptor activation [169, 170]. The roles of the amino acid residues marked in red have been analyzed by Hoffmann et al. [69]. Arg256 and Lys280 are important for ligand recognition.
The crystallography of P2Y12 receptor proteins bound to agonists and antagonists will further facilitate the development of novel P2Y12 receptor ligands [169, 170].
FormalPara P2Y13 receptor ligandsThe P2Y13 receptor is involved in degranulation of mast cells, metabolic effects and neuroprotection [20, 46, 55, 127, 128]. ADP is the principal agonist activating the P2Y13 receptor Table 7, [36]. ATP may act as a partial agonist [106].
Analogues of PPADS (pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid) including MRS2211 and MRS2603 inhibit the activation of the human P2Y13 receptor Table 7; [90]. Cangrelor blocks both P2Y12 receptor and the P2Y13 receptor [52, 106].
FormalPara P2Y14 receptor ligandsThe P2Y14 receptor is involved in inflammation, pain, control of mast cells and microglia, insulin release, and vasoconstriction [1, 13, 39, 56, 110, 117]. UDP and UDP-sugars such as UDP-galactose and UDP-glucose activate the receptor Table 8, [25, 27, 50]. UDP analogues such as 5-iodo-UDP (MRS2690) and α,β-methylene-2-thio-UDP (MRS2905) are potent and selective agonists activating the P2Y14 receptor Table 8, [25, 40, 94].
Potent and selective antagonists include MRS4608 Table 8; [85] and PPTN (4-[4-(4-piperidinyl)phenyl]-7-[4-(trifluoromethyl)phenyl]-2-naphthalenecarboxylic acid; Table 8; [13]. MRS4174 is a fluorescent antagonist with an affinity in the nanomolar range Table 8, [93].
Data availability
“Not applicable”.
References
Abbas ZSB, Latif ML, Dovlatova N, Fox SC, Heptinstall S, Dunn WR, Ralevic V (2018) UDP-sugars activate P2Y14 receptors to mediate vasoconstriction of the porcine coronary artery. Vasc Pharmacol 103–105:36–46
Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341
Ahn YH, Lee JY, Park HD, Kim TH, Park MC, Choi G, Kim S (2016) Identification of a new morpholine scaffold as a P2Y12 receptor antagonist. Molecules 21:E1114
Algaier I, Jakubowski JA, Asai F, von Kügelgen I (2008) Interaction of the active metabolite of prasugrel, R-138727, with cysteine 97 and cysteine 175 of the human P2Y12 receptor. J Thromb Haemost 6:1908–1914
Amadio S, Parisi C, Montilli C, Carrubba AS, Apolloni S, Volonté C (2014) P2Y12 receptor on the verge of a neuroinflammatory breakdown. Mediators Inflamm 2014:975849
Aungraheeta R, Conibear A, Butler M, Kelly E, Nylander S, Mumford A, Mundell SJ (2016) Inverse agonism at the P2Y12 receptor and ENT1 transporter blockade contribute to platelet inhibition by ticagrelor. Blood 128:2717–2728
Ayyanathan K, Webb TE, Sandhu AK, Athwal RS, Barnard EA, Kunapuli SP (1996) Cloning and chromosomal localization of the human P2Y1 purinoceptor. Biochem Biophys Res Commun 218:783–788
Bach P, Boström J, Brickmann K, van Giezen JJ, Groneberg RD, Harvey DM, O’Sullivan M, Zetterberg F (2013) Synthesis, structure-property relationships and pharmacokinetic evaluation of ethyl 6-aminonicotinate sulfonylureas as antagonists of the P2Y12 receptor. Eur J Med Chem 65:360–375
Baldoni B, Bruderer S, Krause A, Gutierrez M, Gueret P, Astruc B, Dingemanse J (2014) A new reversible and potent P2Y12 receptor antagonist (ACT-246475): tolerability, pharmacokinetics, and pharmacodynamics in a first-in-man trial. Clin Drug Investig 34:807–818
Baqi Y, Müller CE (2019) Antithrombotic P2Y12 receptor antagonists: recent developments in drug discovery. Drug Discov Today 24:325–333
Baqi Y, Atzler K, Köse M, Glänzel M, Müller CE (2009) High-affinity, non-nucleotide-derived competitive antagonists of platelet P2Y12 receptors. J Med Chem 52:3784–3793
Barragán-Iglesias P, Mendoza-Garcés L, Pineda-Farias JB, Solano-Olivares V, Rodríguez-Silverio J, Flores-Murrieta FJ, Granados-Soto V, Rocha-González HI (2015) Participation of peripheral P2Y1, P2Y6 and P2Y11 receptors in formalin-induced inflammatory pain in rats. Pharmacol Biochem Behav 128:23–32
Barrett MO, Sesma JI, Ball CB, Jayasekara PS, Jacobson KA, Lazarowski ER, Harden TK (2013) A selective high-affinity antagonist of the P2Y14 receptor inhibits UDP-glucose-stimulated chemotaxis of human neutrophils. Mol Pharmacol 84:41–49
Beavers CJ, Effoe SA, Dobesh PP (2022) Selatogrel: a novel subcutaneous P2Y12 inhibitor. J Cardiovasc Pharmacol 79:161–167
Bednar F, Kroupa J, Ondrakova M, Osmancik P, Kopa M, Motovska Z (2016) Antiplatelet efficacy of P2Y12 inhibitors (prasugrel, ticagrelor, clopidogrel) in patients treated with mild therapeutic hypothermia after cardiac arrest due to acute myocardial infarction. J Thromb Thrombolysis 41:549–555
Ben Addi A, Cammarata D, Conley PB, Boeynaems JM, Robaye B (2010) Role of the P2Y12 receptor in the modulation of murine dendritic cell function by ADP. J Immunol 185:5900–5906
Besada P, Shin DH, Costanzi S, Ko H, Mathé C, Gagneron J, Gosselin G, Maddileti S, Harden TK, Jacobson KA (2006) Structure-activity relationships of uridine 5’-diphosphate analogues at the human P2Y6 receptor. J Med Chem 49:5532–5543
Bijak M, Szelenberger R, Dziedzic A, Saluk-Bijak J (2018) Inhibitory effect of flavonolignans on the P2Y12 pathway in blood platelets. Molecules 23:374
Björquist A, Di Buduo CA, Femia EA, Storey RF, Becker RC, Balduini A, Nylander S, Cattaneo M (2016) Studies of the interaction of ticagrelor with the P2Y13 receptor and with P2Y13-dependent pro-platelet formation by human megakaryocytes. Thromb Haemost 116:1079–1088
Blom D, Yamin TT, Champy MF, Selloum M, Bedu E, Carballo-Jane E, Gerckens L, Luell S, Meurer R, Chin J, Mudgett J, Puig O (2010) Altered lipoprotein metabolism in P2Y(13) knockout mice. Biochim Biophys Acta 1801:1349–1360
Bogdanov YD, Wildman SS, Clements MP, King BF, Burnstock G (1998) Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br J Pharmacol 124:428–430
Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797
Burnstock G, Kennedy C (1985) Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol 16:433–440
Burnstock G, Boeynaems JM (2014) Purinergic signalling and immune cells. Purinergic Signal 10:529–564
Carter RL, Fricks IP, Barrett MO, Burianek LE, Zhou Y, Ko H, Das A, Jacobson KA, Lazarowski ER, Harden TK (2009) Quantification of Gi-mediated inhibition of adenylyl cyclase activity reveals that UDP is a potent agonist of the human P2Y14 receptor. Mol Pharmacol 76:1341–1348
Cattaneo M (2011) The platelet P2Y12 receptor for adenosine diphosphate: congenital and drug-induced defects. Blood 117:2102–2112
Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L, Trill J, Swift A, Aiyar N, Taylor P, Vawter L, Naheed S, Szekeres P, Hervieu G, Scott C, Watson JM, Murphy AJ, Duzic E, Klein C, Bergsma DJ, Wilson S, Livi GP (2000) A G protein-coupled receptor for UDP-glucose. J Biol Chem 275:10767–10771
Chao H, Turdi H, Herpin TF, Roberge JY, Liu Y, Schnur DM, Poss MA, Rehfuss R, Hua J, Wu Q, Price LA, Abell LM, Schumacher WA, Bostwick JS, Steinbacher TE, Stewart AB, Ogletree ML, Huang CS, Chang M, Cacace AM, Arcuri MJ, Celani D, Wexler RR, Lawrence RM (2013) Discovery of 2-(phenoxypyridine)-3-phenylureas as small molecule P2Y1 antagonists. J Med Chem 56:1704–1714
Charlton ME, Williams AS, Fogliano M, Sweetnam PM, Duman RS (1997) The isolation and characterization of a novel G protein-coupled receptor regulated by immunologic challenge. Brain Res 764:141–148
Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK (2004) Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J Pharmacol Exp Ther 311:1038–1043
Cicko S, Lucattelli M, Müller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Dürk T, Zissel G, Boeynaems JM, Sorichter S, Ferrari D, Di Virgilio F, Virchow JC, Lungarella G, Idzko M (2010) Purinergic receptor inhibition prevents the development of smoke-induced lung injury and emphysema. J Immunol 185:688–697
Communi D, Pirotton S, Parmentier M, Boeynaems JM (1995) Cloning and functional expression of a human uridine nucleotide receptor. J Biol Chem 270:30849–30852
Communi D, Parmentier M, Boeynaems JM (1996) Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem Biophys Res Commun 222:303–308
Communi D, Govaerts C, Parmentier M, Boeynaems JM (1997) Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem 272:31969–31973
Communi D, Robaye B, Boeynaems JM (1999) Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol 128:1199–1206
Communi D, Gonzalez NS, Detheux M, Brezillon S, Lannoy V, Parmentier M, Boeynaems JM (2001) Identification of a novel human ADP receptor coupled to Gi. J Biol Chem 276:41479–41485
Conroy S, Kindon ND, Glenn J, Stoddart LA, Lewis RJ, Hill SJ, Kellam B, Stocks MJ (2018) Synthesis and evaluation of the first fluorescent antagonists of the human P2Y2 receptor based on AR-C118925. J Med Chem 61:3089–3113
Crepaldi P, Cacciari B, Bonache MC, Spalluto G, Varani K, Borea PA, von Kügelgen I, Hoffmann K, Pugliano M, Razzari C, Cattaneo M (2009) 6-Amino-2-mercapto-3H-pyrimidin-4-one derivatives as new candidates for the antagonism at the P2Y12 receptors. Bioorg Med Chem 17:4612–4621
Curet MA, Watters JJ (2018) P2Y14 receptor activation decreases interleukin-6 production and glioma GL261 cell proliferation in microglial transwell cultures. J Neurooncol 137:23–31
Das A, Ko H, Burianek LE, Barrett MO, Harden TK, Jacobson KA (2010) Human P2Y14 receptor agonists: truncation of the hexose moiety of uridine-5’-diphosphoglucose and its replacement with alkyl and aryl groups. J Med Chem 53:471–480
Ding Z, Bynagari YS, Mada SR, Jakubowski JA, Kunapuli SP (2009) Studies on the role of the extracellular cysteines and oligomeric structures of the P2Y12 receptor when interacting with antagonists. J Thromb Haemost 7:232–234
El-Tayeb A, Griessmeier KJ, Müller CE (2005) Synthesis and preliminary evaluation of [3H]PSB-0413, a selective antagonist radioligand for platelet P2Y12 receptors. Bioorg Med Chem Lett 15:5450–5452
El-Tayeb A, Qi A, Müller CE (2006) Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors. J Med Chem 49:7076–7087
El-Sayed FG, Camden JM, Woods LT, Khalafalla MG, Petris MJ, Erb L, Weisman GA (2014) P2Y2 nucleotide receptor activation enhances the aggregation and self-organization of dispersed salivary epithelial cells. Am J Physiol Cell Physiol 307:C83-96
Erb L, Lustig KD, Sullivan DM, Turner JT, Weisman GA (1993) Functional expression and photoaffinity labeling of a cloned P2U purinergic receptor. Proc Natl Acad Sci USA 90:10449–10453
Fabre AC, Malaval C, Ben Addi A, Verdier C, Pons V, Serhan N, Lichtenstein L, Combes G, Huby T, Briand F, Collet X, Nijstad N, Tietge UJ, Robaye B, Perret B, Boeynaems JM, Martinez LO (2010) P2Y13 receptor is critical for reverse cholesterol transport. Hepatology 52:1477–1483
Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE et al (2001) Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest 107:1591–1598
Franke H, Illes P (2014) Nucleotide signaling in astrogliosis. Neurosci Lett 565:14–22
Fredriksson R, Lagerström MC, Lundin LG, Schioth HB (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63:1256–1272
Freeman K, Tsui P, Moore D, Emson PC, Vawter L, Naheed S, Lane P, Bawagan H, Herrity N, Murphy K, Sarau HM, Ames RS, Wilson S, Livi GP, Chambers JK (2001) Cloning, pharmacology, and tissue distribution of G-protein-coupled receptor GPR105 (KIAA0001) rodent orthologs. Genomics 78:124–128
Fricks IP, Carter RL, Lazarowski ER, Harden TK (2009) Gi-dependent cell signaling responses of the human P2Y14 receptor in model cell systems. J Pharmacol Exp Ther 330:162–168
Fumagalli M, Trincavelli L, Lecca D, Martini C, Ciana P, Abbracchio MP (2004) Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y13 receptor. Biochem Pharmacol 68:113–124
Gao ZG, Jacobson KA (2017) Distinct signaling patterns of allosteric antagonism at the P2Y1 receptor. Mol Pharmacol 92:613–626
Gao ZG, Mamedova L, Tchilibon S, Gross AS, Jacobson KA (2004) 2,2’-Pyridylisatogen tosylate antagonizes P2Y1 receptor signaling without affecting nucleotide binding. Biochem Pharmacol 68:231–237
Gao ZG, Ding Y, Jacobson KA (2010) P2Y13 receptor is responsible for ADP-mediated degranulation in RBL-2H3 rat mast cells. Pharmacol Res 62:500–505
Gao ZG, Wei Q, Jayasekara MP, Jacobson KA (2013) The role of P2Y14 and other P2Y receptors in degranulation of human LAD2 mast cells. Purinergic Signal 9:31–40
Garcia C, Maurel-Ribes A, Nauze M, N’Guyen D, Martinez LO, Payrastre B, Sénard JM, Galés C, Pons V (2019) Deciphering biased inverse agonism of cangrelor and ticagrelor at P2Y12 receptor. Cell Mol Life Sci 76:561–576
Giannattasio G, Ohta S, Boyce JR, Xing W, Balestrieri B, Boyce JA (2011) The purinergic G protein-coupled receptor 6 inhibits effector T cell activation in allergic pulmonary inflammation. J Immunol 187:1486–1495
Ginsburg-Shmuel T, Haas M, Schumann M, Reiser G, Kalid O, Stern N, Fischer B (2010) 5-OMe-UDP is a potent and selective P2Y6-receptor agonist. J Med Chem 53:1673–1685
Girard M, Bellefeuille SD, Eiselt E, Brouillette R, Placet M, Arguin G, Longpré JM, Sarret P, Gendron FP (2020) The P2Y6 receptor signals through Gαq/Ca2+/PKCα and Gα13/ROCK pathways to drive the formation of membrane protrusions and dictate cell migration. J Cell Physiol 235:9676–9690
Haas M, Shaaban A, Reiser G (2014) Alanine-(87)-threonine polymorphism impairs signaling and internalization of the human P2Y11 receptor, when co-expressed with the P2Y1 receptor. J Neurochem 129:602–613
Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9:1512–1519
Hechler B, Gachet C (2015) Purinergic receptors in thrombosis and inflammation. Arterioscler Thromb Vasc Biol 35:2307–2315
Hechler B, Vigne P, Léon C, Breittmayer JP, Gachet C, Frelin C (1998) ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol Pharmacol 53:727–733
Herbert JM, Savi P (2003) P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med 3:113–122
Hillmann P, Ko GY, Spinrath A, Raulf A, von Kügelgen I, Wolff SC, Nicholas RA, Kostenis E, Höltje HD, Müller CE (2009) Key determinants of nucleotide-activated G protein-coupled P2Y2 receptor function revealed by chemical and pharmacological experiments, mutagenesis and homology modeling. J Med Chem 52:2762–2775
Hoffmann K, Baqi Y, Morena MS, Glänzel M, Müller CE, von Kügelgen I (2009) Interaction of new, very potent non-nucleotide antagonists with Arg256 of the human platelet P2Y12 receptor. J Pharmacol Exp Ther 331:648–655
Hoffmann K, Lutz DA, Straßburger J, Baqi Y, Müller CE, von Kügelgen I (2014) Competitive mode and site of interaction of ticagrelor at the human platelet P2Y12 -receptor. J Thromb Haemost 12:1898–1905
Hoffmann K, Sixel U, Di Pasquale F, von Kügelgen I (2008) Involvement of basic amino acid residues in transmembrane regions 6 and 7 in agonist and antagonist recognition of the human platelet P2Y12-receptor. Biochem Pharmacol 76:1201–1213
Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB (2001) Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409:202–207
Horckmans M, Léon-Gómez E, Robaye B, Balligand JL, Boeynaems JM, Dessy C, Communi D (2012) Gene deletion of P2Y4 receptor lowers exercise capacity and reduces myocardial hypertrophy with swimming exercise. Am J Physiol Heart Circ Physiol 303:H835-843
Horváth G, Gölöncsér F, Csölle C, Király K, Andó RD, Baranyi M, Koványi B, Máté Z, Hoffmann K, Algaier I, Baqi Y, Müller CE, von Kügelgen I, Sperlágh B (2014) Central P2Y12 receptor blockade alleviates inflammatory and neuropathic pain and cytokine production in rodents. Neurobiol Dis 70:162–178
Houston D, Ohno M, Nicholas RA, Jacobson KA, Harden TK (2006) [32P]2-iodo-N6-methyl-(N)-methanocarba-2’-deoxyadenosine-3’,5’-bisphosphate ([32P]MRS2500), a novel radioligand for quantification of native P2Y1 receptors. Br J Pharmacol 147:459–467
IJzerman AP, Jacobson KA, Müller CE, Cronstein BN, Cunha RA (2022) International union of basic and clinical pharmacology. CXII: adenosine receptors: a further update. Pharmacol Rev 74:340–372
Ingall AH, Dixon J, Bailey A, Coombs ME, Cox D, McInally JI, Hunt SF, Kindon ND, Teobald BJ, Willis PA, Humphries RG, Leff P, Clegg JA, Smith JA, Tomlinson W (1999) Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J Med Chem 42:213–220
Ito M, Egashira SI, Yoshida K, Mineno T, Kumagai K, Kojima H, Okabe T, Nagano T, Ui M, Matsuoka I (2017) Identification of novel selective P2Y6 receptor antagonists by high-throughput screening assay. Life Sci 180:137–142
Ivanov AA, Ko H, Cosyn L, Maddileti S, Besada P, Fricks I, Costanzi S, Harden TK, Calenbergh SV, Jacobson KA (2007) Molecular modeling of the human P2Y2 receptor and design of a selective agonist, 2’-amino-2’-deoxy-2-thiouridine 5’-triphosphate. J Med Chem 50:1166–1176
Jacob PF, Vaz SH, Ribeiro JA, Sebastião AM (2014) P2Y1 receptor inhibits GABA transport through a calcium signalling-dependent mechanism in rat cortical astrocytes. Glia 62:1211–1226
Jacob TF, Singh V, Dixit M, Ginsburg-Shmuel T, Fonseca B, Pintor J, Youdim MBH, Major DT, Weinreb O, Fischer B (2018) A promising drug candidate for the treatment of glaucoma based on a P2Y6-receptor agonist. Purinerg Signal 14:271–284
Jacobson KA, Müller CE (2016) Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 104:31–49
Jacobson KA, Ivanov AA, de Castro S, Harden TK, Ko H (2009) Development of selective agonists and antagonists of P2Y receptors. Purinerg Signal 5:75–89
Jacobson KA, Gao ZG, Paoletta S, Kiselev E, Chakraborty S, Jayasekara PS, Balasubramanian R, Tosh DK (2014) John Daly lecture: structure-guided drug design for adenosine and P2Y receptors. Comput Struct Biotechnol J 13:286–298
Jacobson KA, Delicado EG, Gachet C, Kennedy C, von Kügelgen I, Li B, Miras-Portugal MT, Novak I, Schöneberg T, Perez-Sen R, Thor D, Wu B, Yang Z, Müller CE (2020) Update of P2Y receptor pharmacology: IUPHAR Review 27. Br J Pharmacol 177:2413–2433
Janssens R, Communi D, Pirotton S, Samson M, Parmentier M, Boeynaems JM (1996) Cloning and tissue distribution of the human P2Y1 receptor. Biochem Biophys Res Commun 221:588–593
Jung YH, Yu J, Wen Z, Salmaso V, Karcz TP, Phung NB, Chen Z, Duca S, Bennett JM, Dudas S, Salvemini D, Gao ZG, Cook DN, Jacobson KA (2020) Exploration of alternative scaffolds for P2Y14 receptor antagonists containing a biaryl core. J Med Chem 63:9563–9589
Kauffenstein G, Hechler B, Cazenave JP, Gachet C (2004) Adenine triphosphate nucleotides are antagonists at the P2Y12 receptor. J Thromb Haemost 2:1980–1988
Kemp PA, Sugar RA, Jackson AD (2004) Nucleotide-mediated mucin secretion from differentiated human bronchial epithelial cells. Am J Respir Cell Mol Biol 31:446–455
Kennedy C, Qi AD, Herold CL, Harden TK, Nicholas RA (2000) ATP, an agonist at the rat P2Y4 receptor, is an antagonist at the human P2Y4 receptor. Mol Pharmacol 57:926–931
Kennedy C (2021) The P2Y/P2X divide: How it began. Biochem Pharmacol 187:114408
Kim YC, Lee JS, Sak K, Marteau F, Mamedova L, Boeynaems JM, Jacobson KA (2005) Synthesis of pyridoxal phosphate derivatives with antagonist activity at the P2Y13 receptor. Biochem Pharmacol 70:266–274
Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD et al (2003) 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a northern conformation: enhanced potency as P2Y1 receptor antagonists. J Med Chem 46:4974–4987
Kindon N, Davis A, Dougall I, Dixon J, Johnson T, Walters I, Thom S, McKechnie K, Meghani P, Stocks MJ (2017) From UTP to AR-C118925, the discovery of a potent non nucleotide antagonist of the P2Y2 receptor. Bioorg Med Chem Lett 27:4849–4853
Kiselev E, Balasubramanian R, Uliassi E, Brown KA, Trujillo K, Katritch V, Hammes E, Stevens RC, Harden TK, Jacobson KA (2015) Design, Synthesis and Pharmacological Characterization of a Fluorescent Agonist of the P2Y14 Receptor. Bioorg Med Chem Lett 25:4733–4739
Ko H, Fricks I, Ivanov AA, Harden TK, Jacobson KA (2007) Structure-activity relationship of uridine 5’-diphosphoglucose analogues as agonists of the human P2Y14 receptor. J Med Chem 50:2030–2039
Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K (2007) UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446:1091–1095
Lazarowski ER, Watt WC, Stutts MJ, Boucher RC, Harden TK (1995) Pharmacological selectivity of the cloned human P2U-purinoceptor: potent activation by diadenosine tetraphosphate. Br J Pharmacol 116:1619–1627
Leipziger J (2003) Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284:F419-432
Léon C, Vial C, Cazenave JP, Gachet C (1996) Cloning and sequencing of a human cDNA encoding endothelial P2Y1 purinoceptor. Gene 171:295–297
Leonardi S, Bhatt DL (2019) Practical considerations for cangrelor use in patients with acute coronary syndromes. Eur Heart J Acute Cardiovasc Care 8:39–44
Li Z, He C, Zhang J, Zhang H, Wei H, Wu S, Jiang W (2020) P2Y6 Deficiency Enhances Dendritic Cell-Mediated Th1/Th17 Differentiation and Aggravates Experimental Autoimmune Encephalomyelitis. J Immunol 205:387–397
Liu X, Gao ZG, Wu Y, Stevens RC, Jacobson KA, Zhao S (2018) Salvianolic acids from antithrombotic traditional chinese medicine danshen are antagonists of human P2Y1 and P2Y12 receptors. Sci Rep 8:8084
Lustig KD, Shiau AK, Brake AJ, Julius D (1993) Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci USA 90:5113–5117
Malmsjö M, Hou M, Pendergast W, Erlinge D, Edvinsson L (2003) Potent P2Y6 receptor mediated contractions in human cerebral arteries. BMC Pharmacol 3:4
Mamedova LK, Joshi BV, Gao ZG, von Kügelgen I, Jacobson KA (2004) Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol 67:1763–1770
Mansour A, Bachelot-Loza C, Nesseler N, Gaussem P, Gouin-Thibault I (2020) P2Y12 inhibition beyond thrombosis: effects on inflammation. Int J Mol Sci 21:1391
Marteau F, Le Poul E, Communi D, Communi D, Labouret C, Savi P, Boeynaems JM, Gonzalez NS (2003) Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol 64:104–112
Maruoka H, Barrett MO, Ko H, Tosh DK, Melman A, Burianek LE, Balasubramanian R, Berk B, Costanzi S, Harden TK, Jacobson KA (2010) Pyrimidine ribonucleotides with enhanced selectivity as P2Y(6) receptor agonists: novel 4-alkyloxyimino, (S)-methanocarba, and 5’-triphosphate gamma-ester modifications. J Med Chem 53:4488–4501
Maruoka H, Jayasekara MP, Barrett MO, Franklin DA, de Castro S, Kim N, Costanzi S, Harden TK, Jacobson KA (2011) Pyrimidine nucleotides with 4-alkyloxyimino and terminal tetraphosphate δ-ester modifications as selective agonists of the P2Y(4) receptor. J Med Chem 54:4018–4033
Meis S, Hamacher A, Hongwiset D, Marzian C, Wiese M, Eckstein N, Royer HD, Communi D, Boeynaems JM, Hausmann R, Schmalzing G, Kassack MU (2010) NF546 [4,4’-(carbonylbis(imino-3,1-phenylene-carbonylimino-3,1-(4-methyl-phenylene)-carbonylimino))-bis(1,3-xylene-alpha, alpha’-diphosphonic acid) tetrasodium salt] is a non-nucleotide P2Y11 agonist and stimulates release of interleukin-8 from human monocyte-derived dendritic cells. J Pharmacol Exp Ther 332:238–247
Meister J, Le Duc D, Ricken A, Burkhardt R, Thiery J, Pfannkuche H, Polte T, Grosse J, Schöneberg T, Schulz A (2014) The G protein-coupled receptor P2Y14 influences insulin release and smooth muscle function in mice. J Biol Chem 289:23353–23366
Miras-Portugal MT, Gualix J, Pintor J (1998) The neurotransmitter role of diadenosine polyphosphates. FEBS Lett 430:78–82
Miras-Portugal MT, Gomez-Villafuertes R, Gualix J, Diaz-Hernandez JI, Artalejo AR, Ortega F, Delicado EG, Perez-Sen R (2016) Nucleotides in neuroregeneration and neuroprotection. Neuropharmacology 104:243–254
Miras-Portugal MT, Queipo MJ, Gil-Redondo JC, Ortega F, Gómez-Villafuertes R, Gualix J, Delicado EG, Pérez-Sen R (2019) P2 receptor interaction and signalling cascades in neuroprotection. Brain Res Bull 151:74–83
Miras-Portugal MT, Ortega F, Gómez-Villafuertes R, Gualix J, Pérez-Sen R, Delicado EG (2021) P2X7 receptors in the central nervous system. Biochem Pharmacol 187:114472
Mitchell C, Syed NI, Tengah A, Gurney AM, Kennedy C (2012) Identification of contractile P2Y1, P2Y6, and P2Y12 receptors in rat intrapulmonary artery using selective ligands. J Pharmacol Exp Ther 343:755–762
Moldovan RP, Wenzel B, Teodoro R, Neumann W, Dukic-Stefanovic S, Kraus W, Rong P, Deuther-Conrad W, Hey-Hawkins E, Krügel U, Brust P (2019) Studies towards the development of a PET radiotracer for imaging of the P2Y1 receptors in the brain: synthesis, 18F-labeling and preliminary biological evaluation. Eur J Med Chem 165:142–159
Mufti F, Jung YH, Giancotti LA, Yu J, Chen Z, Phung NB, Jacobson KA, Salvemini D (2020) P2Y14 receptor antagonists reverse chronic neuropathic pain in a mouse model. ACS Med Chem Lett 11:1281–1286
Müller CE, Namasivayam V (2021) Recommended tool compounds and drugs for blocking P2X and P2Y receptors. Purinergic Signal 17:633–648
Müller CE, Baqi Y, Namasivayam V (2020) Agonists and Antagonists for Purinergic Receptors. Methods Mol Biol 2041:45–64
Müller CE (2002) P2-pyrimidinergic receptors and their ligands. Curr Pharm Des 8:2353–2369
Neumann A, Müller CE, Namasivayam V (2020) P2Y1-like nucleotide receptors—Structures, molecular modeling, mutagenesis, and oligomerization. WIREs Comput Mol Sci 10:e1464
Nguyen T, Erb L, Weisman GA, Marchese A, Heng HH, Garrad RC et al (1995) Cloning, expression, and chromosomal localization of the human uridine nucleotide receptor gene. J Biol Chem 270:30845–30848
Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden K (1996) Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol 50:224–229
Ohlmann P, de Castro S, Brown GG, Gachet C, Jacobson KA, Harden TK (2010) Quantification of recombinant and platelet P2Y1 receptors utilizing a [125I]-labeled high-affinity antagonist 2-iodo-N6-methyl-(N)-methanocarba-2’-deoxyadenosine-3’,5’-bisphosphate ([125I]MRS2500). Pharmacol Res 62:344–351
Ohlmann P, Lecchi A, El-Tayeb A, Müller CE, Cattaneo M, Gachet C (2013) The platelet P2Y(12) receptor under normal and pathological conditions. Assessment with the radiolabeled selective antagonist [(3)H]PSB-0413. Purinergic Signal 9:59–66
Olivier CB, Diehl P, Schnabel K, Weik P, Zhou Q, Bode C, Moser M (2014) Third generation P2Y12 antagonists inhibit platelet aggregation more effectively than clopidogrel in a myocardial infarction registry. Thromb Haemost 111:266–272
Orriss I, Syberg S, Wang N, Robaye B, Gartland A, Jorgensen N, Arnett T, Boeynaems JM (2011) Bone phenotypes of P2 receptor knockout mice. Front Biosci 3:1038–1046
Ortega F, Pérez-Sen R, Delicado EG, Miras-Portugal MT (2011) ERK1/2 activation is involved in the neuroprotective action of P2Y13 and P2X7 receptors against glutamate excitotoxicity in cerebellar granule neurons. Neuropharmacology 61:1210–1221
Paoletta S, Sabbadin D, von Kügelgen I, Hinz S, Katritch V, Hoffmann K, Abdelrahman A, Straßburger J, Baqi Y, Zhao Q, Stevens RC, Moro S, Müller CE, Jacobson KA (2015) Modeling ligand recognition at the P2Y12 receptor in light of X-ray structural information. J Comput Aided Mol Des 29:737–756
Parlow JJ, Burney MW, Case BL, Girard TJ, Hall KA, Harris PK, Hiebsch RR, Huff RM, Lachance RM, Mischke DA, Rapp SR, Woerndle RS, Ennis MD (2010) Piperazinyl glutamate pyridines as potent orally bioavailable P2Y12 antagonists for inhibition of platelet aggregation. J Med Chem 53:2010–2037
Pendergast W, Yerxa BR, Douglass JG, Shaver SR, Dougherty RW, Redick CC et al (2001) Synthesis and P2Y receptor activity of a series of uridine dinucleoside 5’-polyphosphates. Bioorg Med Chem Lett 11:157–160
Pons V, Garcia C, Tidten-Luksch N, Mac Sweeney A, Caroff E, Galés C, Riederer MA (2022) Inverse agonist efficacy of selatogrel blunts constitutive P2Y12 receptor signaling by inducing the inactive receptor conformation. Biochem Pharmacol 206:115291
Potthoff SA, Stegbauer J, Becker J, Wagenhaeuser PJ, Duvnjak B, Rump LC, Vonend O (2013) P2Y2 receptor deficiency aggravates chronic kidney disease progression. Front Physiol 4:234
Quintas C, Pinho D, Pereira C, Saraiva L, Gonçalves J, Queiroz G (2014) Microglia P2Y6 receptors mediate nitric oxide release and astrocyte apoptosis. J Neuroinflammation 11:141
Rafehi M, Müller CE (2018) Tools and drugs for uracil nucleotide-activated P2Y receptors. Pharmacol Ther 190:24–80
Rafehi M, Neumann A, Baqi Y, Malik EM, Wiese M, Namasivayam V, Müller CE (2017) Molecular recognition of agonists and antagonists by the nucleotide-activated G protein-coupled P2Y2 receptor. J Med Chem 60:8425–8440
Rafehi M, Malik EM, Neumann A, Abdelrahman A, Hanck T, Namasivayam V, Müller CE, Baqi Y (2017) Development of potent and selective antagonists for the UTP-activated P2Y4 receptor. J Med Chem 60:3020–3038
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Ralevic V, Burnstock G (2003) Involvement of purinergic signaling in cardiovascular diseases. New Drug News Perspect 16:133–140
Relvas LJ, Makhoul M, Dewispelaere R, Caspers L, Communi D, Boeynaems JM, Robaye B, Bruyns C, Willermain F (2015) P2Y2R deficiency attenuates experimental autoimmune uveitis development. PLoS ONE 10:e0116518
Rieg T, Gerasimova M, Boyer JL, Insel PA, Vallon V (2011) P2Y2 receptor activation decreases blood pressure and increases renal Na excretion. Am J Physiol Regul Integr Comp Physiol 301:R510-518
Robaye B, Ghanem E, Wilkin F, Fokan D, Van Driessche W, Schurmans S, Boeynaems JM, Beauwens R (2003) Loss of nucleotide regulation of epithelial chloride transport in the jejunum of P2Y4-null mice. Mol Pharmacol 63:777–783
Salmaso V, Jacobson KA (2020) In silico drug design for purinergic GPCRs: Overview on molecular dynamics applied to adenosine and P2Y receptors. Biomolecules 10:812
Savi P, Zachayus JL, Delesque-Touchard N, Labouret C, Hervé C, Uzabiaga MF, Pereillo JM, Culouscou JM, Bono F, Ferrara P, Herbert JM (2006) The active metabolite of clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci USA 103:11069–11074
Sil P, Hayes CP, Reaves BJ, Breen P, Quinn S, Sokolove J, Rada B (2017) P2Y6 receptor antagonist MRS2578 inhibits neutrophil activation and aggregated neutrophil extracellular trap formation induced by gout-associated monosodium urate crystals. J Immunol 198:428–442
Springthorpe B, Bailey A, Barton P, Birkinshaw TN, Bonnert RV, Brown RC et al (2007) From ATP to AZD6140: the discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorg Med Chem Lett 17:6013–6018
Storey RF, Gurbel PA, Berg JT, Bernaud C, Dangas GD, Frenoux JM, Gorog DA, Hmissi A, Kunadian V, James SK, Tanguay JF, Tran H, Trenk D, Ufer M, Van der Harst P, Van’t Hof AW, Angiolillo DJ (2020) Pharmacodynamics, pharmacokinetics, and safety of single-dose subcutaneous administration of selatogrel, a novel P2Y12 receptor antagonist, in patients with chronic coronary syndromes. Eur Heart J 41:3132–3140
Su X, Floyd DH, Hughes A, Xiang J, Schneider JG, Uluckan O, Heller E, Deng H, Zou W, Craft CS, Wu K, Hirbe AC, Grabowska D, Eagleton MC, Townsley S, Collins L, Piwnica-Worms D, Steinberg TH, Novack DV, Conley PB, Hurchla MA, Rogers M, Weilbaecher KN (2012) The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J Clin Invest 122:3579–3592
Sugidachi A, Asai F, Ogawa T, Inoue T, Koike H (2000) The in vivo pharmacological profile of CS-747, a novel antiplatelet agent with platelet ADP receptor antagonist properties. Br J Pharmacol 129:1439–1446
Sugidachi A, Mizuno M, Ohno K, Jakubowski JA, Tomizawa A (2016) The active metabolite of prasugrel, R-138727, improves cerebral blood flow and reduces cerebral infarction and neurologic deficits in a non-human primate model of acute ischaemic stroke. Eur J Pharmacol 788:132–139
Suzuki T, Kohyama K, Moriyama K, Ozaki M, Hasegawa S, Ueno T, Saitoe M, Morio T, Hayashi M, Sakuma H (2020) Extracellular ADP augments microglial inflammasome and NF-κB activation via the P2Y12 receptor. Eur J Immunol 50:205–219
Takasaki J, Kamohara M, Saito T, Matsumoto M, Matsumoto S, Ohishi T, Soga T, Matsushime H, Furuichi K (2001) Molecular cloning of the platelet P2TAC ADP receptor: pharmacological comparison with another ADP receptor, the P2Y1 receptor. Mol Pharmacol 60:432–439
Tauber J, Davitt WF, Bokosky JE, Nichols KK, Yerxa BR, Schaberg AE, LaVange LM, Mills-Wilson MC, Kellerman DJ (2004) Double-masked, placebo-controlled safety and efficacy trial of diquafosol tetrasodium (INS365) ophthalmic solution for the treatment of dry eye. Cornea 23:784–792
Ullmann H, Meis S, Hongwiset D, Marzian C, Wiese M, Nickel P, Communi D, Boeynaems JM, Wolf C, Hausmann R, Schmalzing G, Kassack MU (2005) Synthesis and structure-activity relationships of suramin-derived P2Y11 receptor antagonists with nanomolar potency. J Med Chem 48:7040–7048
van Giezen JJ, Nilsson L, Berntsson P, Wissing BM, Giordanetto F, Tomlinson W, Greasley PJ (2009) Ticagrelor binds to human P2Y12 independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemost 7:1556–1565
von Kügelgen I (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110:415–432
von Kügelgen I (2019) Pharmacology of P2Y receptors. Brain Res Bull 151:12–24
von Kügelgen I, Harden TK (2011) Molecular pharmacology, physiology, and structure of the P2Y receptors. Adv Pharmacol 61:373–415
von Kügelgen I, Hoffmann K (2016) Pharmacology and structure of P2Y receptors. Neuropharmacology 104:50–61
von Kügelgen I (2021) Molecular pharmacology of P2Y receptor subtypes. Biochem Pharmacol 187:114361
Waldo GL, Harden TK (2004) Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol 65:426–436
Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji XD et al (2002) Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol Pharmacol 62:1249–1257
Wihlborg AK, Wang L, Braun OO, Eyjolfsson A, Gustafsson R, Gudbjartsson T, Erlinge D (2004) ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol 24:1810–1815
Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B (2001) The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol 166:7172–7177
Yamane M, Ogawa Y, Fukui M, Kamoi M, Saijo-Ban Y, Yaguchi S, Mukai S, Kawakita T, Simmura S, Tsubota K (2015) Long-term rebamipide and diquafosol in two cases of immune-mediated dry eye. Optom Vis Sci 92:S25-32
Zambon AC, Brunton LL, Barrett KE, Hughes RJ, Torres B, Insel PA (2001) Cloning, expression, signaling mechanisms, and membrane targeting of P2Y11 receptors in Madin Darby canine kidney cells. Mol Pharmacol 60:26–35
Zech G, Hessler G, Evers A, Weiss T, Florian P, Just M, Czech J, Czechtizky W, Görlitzer J, Ruf S, Kohlmann M, Nazare M (2012) Identification of high-affinity P2Y12 antagonists based on a phenylpyrazole glutamic acid piperazine backbone. J Med Chem 55:8615
Zhang FL, Luo L, Gustafson E, Lachowicz J, Smith M, Qiao X, Liu YH, Chen G, Pramanik B, Laz TM, Palmer K, Bayne M, Monsma FJ (2001) ADP is the cognate ligand for the orphan G protein-coupled receptor SP1999. J Biol Chem 276:8608–8615
Zhang K, Zhang J, Gao ZG, Zhang D, Zhu L, Han GW, Moss SM, Paoletta S, Kiselev E, Lu W, Fenalti G, Zhang W, Müller CE, Yang H, Jiang H, Cherezov V, Katritch V, Jacobson KA, Stevens RC, Wu B, Zhao Q (2014) Structure of the human P2Y12 receptor in complex with an antithrombotic drug. Nature 509:115–118
Zhang J, Zhang K, Gao ZG, Paoletta S, Zhang D, Han GW, Li T, Ma L, Zhang W, Müller CE, Yang H, Jiang H, Cherezov V, Katritch V, Jacobson KA, Stevens RC, Wu B, Zhao Q (2014) Agonist-bound structure of the human P2Y12 receptor. Nature 509:119–122
Zhang D, Gao ZG, Zhang K, Kiselev E, Crane S, Wang J, Paoletta S, Yi C, Ma L, Zhang W, Han GW, Liu H, Cherezov V, Katritch V, Jiang H, Stevens RC, Jacobson KA, Zhao Q, Wu B (2015) Two disparate ligand-binding sites in the human P2Y1 receptor. Nature 520:317–321
Zimmermann H (2021) History of ectonucleotidases and their role in purinergic signaling. Biochem Pharmacol 187:114322
Funding
Open Access funding enabled and organized by Projekt DEAL. Medical faculty of the University of Bonn.
Author information
Authors and Affiliations
Contributions
Ivar von Kügelgen wrote the manuscript and prepared the figure.
Corresponding author
Ethics declarations
Ethical approval
“Not applicable”.
Competing interests
No.
Conflict of interest
Ivar von Kügelgen declares that he has no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
von Kügelgen, I. Pharmacological characterization of P2Y receptor subtypes – an update. Purinergic Signalling 20, 99–108 (2024). https://doi.org/10.1007/s11302-023-09963-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-023-09963-w