Abstract
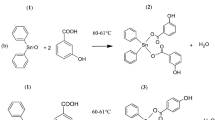
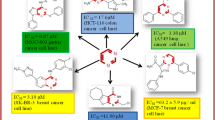
Six 2-(2-acylaminobenzothiazol-6-yl)isoquinoline-1,3(2H,4H)-diones (1a–1f) and five 2-arylisoquinoline-1,3(2H,4H)-diones (1g–1k) were synthesized by refluxing homophthalic anhydrides with 2-acylaminobenzothiazolyl-6-amine or substituted aniline in glacial acetic acid. The cytotoxic activities of 1a–1k were evaluated via MTT method against A431, A549, and PC3. Compound 1b relatively displayed a higher cytotoxic activity than the others. The antitumor effect of 1b were evaluated in established nude mice PANC-1 xenograft model. The results suggest that compound 1b could potentially inhibit tumor growth.



Similar content being viewed by others
References
Bhakta C (1986) Claisen condensation of phthalides with diethyl oxalate formation of 3-carbethoxy-4-hydroxyisocoumarins via rearrangement. Indian J Chem Sect B 25B:189–190
Caputo R, Calabro ML, Micale N, Schimmer AD, Ali M, Zappala M, Grasso S (2012) Synthesis of benzothiazole derivatives and their biological evaluation as anticancer agents. Med Chem Res 21:2644–2651
Cheng CY, Tsai HB, Lin MS (1995) Synthetic approaches to 2-substituted 1-oxo- and 3-oxotetrahydroisoquinolines. J Heterocycl Chem 32:73–77
D’Angelo ND, Kim TS, Andrews K, Booker SK, Caenepeel CK, D’Amico D, Freeman D, Jiang J, Liu L, McCarter JD, Miguel TS, Mullady EL, Schrag M, Subramanian R, Tang J, Wahl ORC, Wang L, Whittington DA, Wu T, Xi N, Xu Y, Yakowec P, Yang OK, Zalameda LP, Zhang N, Hughes P, Norman MH (2011) Discovery and optimization of a series of benzothiazole phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors. J Med Chem 54:1789–1811
Khokra SL, Arora K, Mehta H, Aggarwal A, Yadav M (2011) Common method to synthesize benzothiazole derivatives and their medicinal significance: a review. Int J Pharm Sci Res 2:748–769
Komoda M, Kakuta H, Takahashi H, Fujimoto Y, Kadoya S, Kato F, Hashimoto Y (2001) Specific inhibitor of puromycin-sensitive aminopeptidase with a homophthalimide skeleton: identification of the target molecule and a structure–activity relationship study Bioorg. Med Chem 9:121–131
Matstuda F, Kawasaki M, Ohsaki M, Yamada K, Terashima S (1988) Synthetic studies on nogalamycin congeners [4] syntheses and antitumor activity of various nogalamycin congeners. Tetrahedron 44:5745–5759
Murthy ARK, Hall IH, Chapman JMJ, Rhyne KA, Wyrick SD (1986) The hypolipidemic activity of a series of 2,3-dihydrophthalazine-1,4-dione derivatives in rodent. Pharm Res 3:93–101
Nakagawa A, Uno S, Makishima M, Miyachi H, Hashimoto Y (2008) Progesterone receptor antagonists with a 3-phenylquinazoline-2,4-dione/2-phenylisoquinoline-1,3-dione skeleton. Bioorg Med Chem 16:7046–7054
Nishijima K, Shinkaw T, It M, Nishid H, Yamamotoa I, Onuki Y, Inab H, Miyanob S (1998) Synthesis and diuretic activity of 4,5-dihydro-6H-imidazo[4,5,1-ij] quinoline-6-one-6-oxime-O-sulfonic acid derivatives. Eur J Med Chem 33:763–774
Noguchi T, Sano H, Shimazawa R, Tanatani A, Miyachi H, Hashimoto Y (2004) Phenylhomophthalimide-type NOS inhibitors derived from thalidomide. Bioorg Med Chem Lett 14:4141–4145
Roth J, Madoux F, Hodder P, Roush WR (2008) Synthesis of small molecule inhibitors of the orphan nuclear receptor steroidogenic factor-1 (NR5A1) based on isoquinolinone scaffolds. Bioorg Med Chem Lett 15:2628–2632
Seitz W, Geneste H, Backfisch G, Delzer J, Graef C, Hornberger W, Kling A, Subkowski T, Zimmermann N (2008) Design and synthesis of novel potent and selective integrin αvβ3 antagonists-novel synthetic routes to isoquinolinone, benzoxazinone, and quinazolinone acetates. Bioorg Med Chem Lett 18:527–531
Semple JE, Rydzewski RM, Gardner G (1996) An efficient synthetic route to ethyl 2-aryl-4-hydroxy-1,3(2H,4H)-dioxoisoquinoline-4-carboxylates. J Org Chem 61:7967–7972
Shi XH, Wang Z, Xia Y, Ye TH, Deng M, Xu YZ, Wei Yu-Q, Yu LT (2012) Synthesis and biological evaluation of novel benzothiazole-2-thiol derivatives as potential anticancer agents. Molecules 17:3933–3944
Ukita T, Nakamura Y, Kubo A, Yamamoto Y, Moritani Y, Saruta K, Higashijima T, Kotera J, Takagi M, Kikkawa K, Omori K (2001) Novel, potent, and selective phosphodiesterase5 inhibitors: synthesis and biological activities of a series of 4-aryl-1-isoquinolinone derivatives. J Med Chem 44:2204–2218
Xuan W, Ding W, Hui HX, Zhang SQ (2013) Synthesis and cytotoxic activity of diaryl urea derivatives with a 4-methylpiperazinylcarbonyl moiety. Med Chem Res 22:3857–3862
Yoshida M, Hayakawa I, Hayashi N, Agatsuma T, Oda Y, Tanzawa F, Iwasaki S, Koyama K, Furukawa H, Kurakat S, Sugano Y (2005) Synthesis and biological evaluation of benzothiazole derivatives as potent antitumor agents. Bioorg Med Chem Lett 15:3328–3332
Youssef MM, Amin MA (2010) Microwave assisted synthesis of some new heterocyclic spiro-derivatives with potential antimicrobial and antioxidant activity. Molecules 15:8827–8840
Acknowledgments
Financial support from The National Natural Science Foundation of China (Grant No. 21072156) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, BR., Wang, J., Li, H. et al. Synthesis and antitumor activity evaluation of 2-arylisoquinoline-1,3(2H,4H)-diones in vitro and in vivo. Med Chem Res 23, 1340–1349 (2014). https://doi.org/10.1007/s00044-013-0734-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0734-x