Abstract
Eusocial insects are characterised by a reproductive caste differentiation such that egg-laying is restricted to a small number of queens. The majority of the colony members function as non-reproducing workers and gain indirect fitness by rearing the offspring of the reproductives. In primitively eusocial species, some workers can also get direct fitness by sneaking in some eggs in the presence of the queens, replacing the queens and becoming new queens, or initiating new nests. Here we aimed to understand the factors that permit some workers to gain direct fitness and alter the number of workers doing so, using the primitively eusocial wasp Ropalidia marginata. We transplanted 12 naturally occurring colonies into large laboratory cages where there was adequate space for the workers to leave their natal nests and initiate new colonies. We compared six control colonies that we provided with ad libitum food placed near the nest to six test colonies in which we hand-fed the wasps in addition to the ad libitum food. Wasps in test colonies consumed more food, showed more aggression, replaced their queens, and initiated new nests significantly more often than those in control colonies. When considering all 12 colonies, the rates of queen replacement and nest foundation were significantly positively correlated with food consumption rates. The additional nutrition gained by hand-fed wasps appears to help workers in test colonies to develop their ovaries and lay eggs, implying that they are nutritionally castrated in control colonies.
Similar content being viewed by others
Introduction
The differentiation of colony members into reproductive and non-reproductive castes is one of the defining features of eusociality (Wilson 1971). It is now widely accepted that the evolution of the non-reproductive worker caste is facilitated by the indirect fitness gained by helping closely related reproductive castes and their offspring (Gadau and Fewell 2009). In primitively eusocial species, however, workers can reproduce in the future, either by replacing the present queens of their colonies or by leaving their nests to found new nests (Gadagkar 2001; Brahma et al. 2019; Southon et al. 2020). In a few species of primitively eusocial bees and wasps, workers also lay eggs in the presence of the queen (Gadagkar and Joshi 1982; Tsuchida et al. 2003, 2020; Leadbeater et al. 2011; Andrade et al. 2016). This implies that in addition to indirect fitness gained at present, workers can also gain direct fitness in the future (Ross and Matthews 1991).
The possibility of direct fitness gains by workers is one of the major reasons behind queen-worker conflicts in many primitively eusocial insect societies that are characterised by a strong reproductive skew with one or a few individuals monopolising the reproduction (Field and Cant 2009). Low genetic relatedness between queens and workers, which can be due to polyandrous queens or high rates of queen replacements (Gadagkar et al. 1993; Heinze 2004), is an important reason for such reproductive conflicts. In some species of primitively eusocial bees and wasps, unrelated individuals are present in a colony, and they share a fraction of the direct fitness benefits along with the dominant reproductive individual(s) (Gadagkar et al. 1993; Queller et al. 2000; Leadbeater et al. 2011; Andrade et al. 2016). The age of the workers (Strassmann and Meyer 1983; Field et al. 1999; Sumner et al. 2010; Bang and Gadagkar 2012; Taylor et al. 2020), their position in the colony’s dominance hierarchy (Molina and O’Donnell 2008a; Unnikrishnan and Gadagkar 2017; Fiocca et al. 2020a) and their nutritional status (Gadagkar et al. 1991; Hunt 1991; Molina and O’Donnell 2008a; Shukla et al. 2013; Brahma et al. 2018a; Fiocca et al. 2020a) are known to influence their chances of becoming future reproductives and gaining direct fitness, either on their natal nests or by founding new nests. With this knowledge, we should be able to manipulate one or more of these factors and correspondingly increase or decrease the probabilities that workers will gain direct fitness by replacing their queens or leaving to found new nests.
Among the factors affecting future direct fitness gains by workers, we focussed on the nutritional status of the workers, especially because this can be easily modified. In social insects, nutritional intake in adults influences their reproductive physiology (Hunt 1991; Shukla et al. 2013; O’Donnell et al. 2018). Studies in primitively eusocial wasps have shown that adult nutrition affects the dominance status of workers (Tibbetts and Curtis 2007; Fiocca et al. 2020b) and their potential to become a reproductive in the future (Brahma et al. 2018a; Fiocca et al. 2020b). According to the dominance-nutrition hypothesis (Markiewicz and O’Donnell 2001), dominant individuals develop their ovaries by avoiding energy-intensive work such as foraging, which are left to the subordinate individuals (Molina and O’Donnell 2009; Brahma et al. 2018b). One way to give subordinate individuals a chance of gaining direct fitness is through supplemental feeding of the colony, which we hypothesise will lead to an increased frequency of workers opting to gain direct fitness. This hypothesis has not been adequately tested yet, although it is known that adult nutrition is an important factor in determining the future reproductive potential of workers. Here we tested this hypothesis by experimentally augmenting adult nutrition. For this, we used colonies of the tropical, primitively eusocial wasp, Ropalidia marginata, which has a perennial colony cycle and where workers regularly replace their queens in a conflict-free system of queen succession (Bhadra and Gadagkar 2008; Gadagkar 2017). Upon the natural death or experimental removal of the queen, one and only one worker becomes temporarily hyper-aggressive and assumes the queen position within about a week. Pre-determined potential queens in waiting are not challenged by others and also frequently leave their natal nests, either solitarily or in groups, to found new colonies of their own (Gadagkar 2001, 2009; Brahma et al. 2019). However, unlike in some other primitively eusocial bees and wasps, workers in R. marginata colonies do not lay eggs in the presence of the queen (Gadagkar 2001). Although only queens have fully developed ovaries with mature eggs, some workers can also have slight ovarian development in presence of the queen (Gadagkar 2001). A previous study showed that adults that consumed more food had better developed ovaries (Shukla et al. 2013).
Materials and methods
Experimental set-up
We collected twelve naturally occurring post-emergence nests of R. marginata (see Table S1 for details of the transplanted parent nests), including all adults and brood, from Bangalore (12.97°N, 77.59°E), India and transplanted each into a separate walk-in cage (dimensions: 2.13 m × 2.13 m × 2.13 m). We have previously shown that both queen turnovers and new nest foundations can be studied conveniently in these large walk-in cages (Brahma et al. 2019). We conducted the study in two parts, the first part from February to June 2018 and the second part from February to July 2019. For both the years, all nests were collected in the months of February and March and were transplanted inside the walk-in cages. We uniquely marked every female wasp using quick-drying Testors© enamel paints and maintained nest-maps to record the composition and position of brood at the time of transplantation. We marked newly eclosed individuals every day and recorded their age and updated the nest-maps every week. We provided an ad libitum supply of larvae of the rice moth Corcyra cephalonica, dilute honey and water in each cage at a single feeding station and replenished them every day. We also provided pieces of soft wood as a building material. We made a census of the adult wasps every evening between 2000 and 2100 h to record all the live wasps and their resting positions relative to the transplanted parent nest or any newly initiated nests inside the walk-in cages. In each nest, we identified the queen based on her egg-laying. We measured the life span of a parent nest as the number of days elapsed between our transplanting the nest into the walk-in cage and the nest being abandoned by all the wasps.
Data collection
Each year we randomly designated three colonies as ‘control’ and three as ‘test’. While we supplied the control nests with ad libitum food as described above, we additionally hand-fed the wasps in the test nests. In the test nests, we offered Corcyra cephalonica larvae, using a forceps, to every adult wasp until it refused to take any more, and repeated this procedure twice a day, once in the morning (between 0800 and 1000 h) and once during the late afternoon (between 1500 and 1700 h). Since we hand-fed every wasp in the test colonies until it refused to take any more, it was impossible to have any kind of sham control.
In both control and test nests, we counted the number of C. cephalonica larvae remaining unconsumed every day at the feeding station and calculated the number consumed as the difference between this and the number provided on the previous day (for the control nests) and added this to the number of hand-fed larvae in the test nests. We divided the total number of larvae consumed each day by the number of wasps present on that day to obtain the per-capita food consumption for that day and then averaged over all days of the experiment. In 2018, we collected 2.5 h of quantitative behavioural data, twice a week, in each walk-in cage, focusing on all individual wasps, whether on the parent nest, on a newly initiated nest or somewhere in between. In each such 2.5 h period, we randomly intermingled 13, five minute bouts of manually recording all occurrences of selected behaviours, including dominance-subordinate behaviours, and 12 scans during which we recorded the behavioural state of all individuals at that instant (Gadagkar 2001). The behaviour recordings were made between 0800 and 1800 h, when the wasps are most active (Gadagkar 2001). We continued the behavioural data collection until all wasps abandoned the parent nest. We monitored the cages daily for new nest initiations with at least one egg in them. We monitored all parent nests to record queen turnovers, i.e., when the previous queen was absent and a new individual had been observed to lay at least one egg. We calculated per-capita rates of queen turnover and new nest initiations by dividing the number of respective events by the total number of wasps seen on the parent nests during the course of the experiment.
Data analyses
Using R-Studio (version 3.6.0), we fitted linear regression models to (a) compare control and test colonies with respect to colony duration, per-capita food consumption, queen turnovers and new nest initiations, and (b) to examine the effect of per-capita food consumption on colony tenure, queen turnover, new nest initiations and dominance behaviour. We included the year of the experiment as a predictor variable in all the fitted models. We used the “DHARMa” package (version 0.3.3.0) in R to check for the overdispersion and non-uniformity of residuals in the fitted models in (a) and (b).
Results
At the time of transplantation, all the 12 R. marginata colonies were in their post-emergence phases with a median of 214.5 cells (median; control = 214.5, test = 209.5; Table S1), 130.5 brood which included eggs, five larval instars and pupae (median; control = 129.5, test = 148; Table S1) and 39.5 females (median; control = 42.5, test = 39; Table S1). Control and test colonies did not significantly differ in terms of colony size (Mann Whitney-U test; p = 0.69), amount of brood (Mann Whitney-U test; p = 0.19) or number of females (Mann Whitney-U test; p = 0.34) at the time of transplantation.
Test nests, where the wasps were hand-fed in addition to being supplied with ad libitum food at the feeding station, consumed significantly more food than control nests, in which the wasps only had access to the ad libitum supply of food (linear model; p < 0.0001; Fig. 1A; Table S2). On a per-capita basis, the wasps in the test nests consumed on average 1.9-fold more food than those in the control nests. The life spans of the test nests (mean ± s.d. = 65.8 ± 17.7 days) were significantly shorter than those of the control nests (109.2 ± 10.6) (linear model; p = 0.0009); Fig. 1B; Table S2).
Comparisons between test and control nests. All comparisons are done using linear models and are significant at α = 0.05. a average per-capita food consumption (p < 0.0001); b parent colony duration (p = 0.0009); c number of queen turnovers (p < 0.0001); d per-capita queen turnover (p < 0.0001); e total number of new nests initiated (p = 0.001); f per-capita nest initiation (p = 0.009)
We recorded a total of 40 queen turnover events, of which 37 occurred in the test nests and three occurred in the control nests. Both total and per-capita numbers of queen turnovers were significantly higher in test nests compared to the control nests (total: linear model; p < 0.0001; Fig. 1C; per-capita: linear model; p < 0.0001; Fig. 1D; Table S2). We recorded the initiation of 40 new nests, of which 30 were initiated by wasps leaving the test nests and ten by wasps leaving the control nests. Once again, both total and per-capita nest initiation were significantly greater in test nests compared to the control nests (total: linear model; p = 0.001; Fig. 1E; per-capita: linear model; p = 0.009; Fig. 1F; Table S2). Year of experiment did not have any significant effect on any of the response variables.
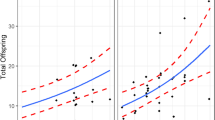
Considering all test and control nests together, the life span of the parent nests decreased significantly with increasing per-capita food consumption (linear model; p = 0.01; Fig. 2A; Table S3). The number of queen turnover events, both total (linear model; p = 0.01; Fig. 2B; Table S3) and per-capita (linear model; p = 0.0005; Fig. 2C; Table S3), increased significantly with increasing per-capita food consumption. Similar trends were shown by the total (linear model; p = 0.007; Fig. 2D; Table S3) and per-capita numbers (linear model; p = 0.0004; Fig. 2E; Table S3) of new nest initiation. Frequency per hour of dominance behaviour shown on the parent nests also increased significantly with the per-capita food consumption in the by the individuals on the parent nests (linear model; p = 0.02; Fig. 2F; Table S3). None of the fitted linear models were overdispersed or had non-uniform residuals.
Change in different response variables with change in average per-capita food consumption. Fitted linear models are significant at α = 0.05. a parent colony duration (p = 0.01); b number of queen turnovers (p = 0.01); c per-capita queen turnover (p = 0.0005); d total number of new nests initiated (p = 0.007); e per-capita nest initiation (p = 0.0004); f frequency per hour of dominance behaviour (p = 0.02)
Discussion
In this study, we used a previously established process of transplanting natural colonies of R. marginata into large walk-in cages (Brahma et al. 2019) to investigate whether more nutrition would reduce the levels of cooperative behaviour in the colonies. We compared control colonies where we provided ad libitum food kept at a feeding station nearby with test colonies in which we hand-fed all adult wasps to satiation every day, in addition to the ad libitum food we provided at the feeding station. Test nests had a significantly shorter life span than control nests; this is not surprising because compared to the control nests, many more individuals left the test nests to become nest foundresses and initiate new nests. As predicted, we found that excess nutrition disrupted the level of cooperation between the reproductive and non-reproductive members of the colony, such that there was an increase in queen-turnovers and of workers deserting their nests to initiate new nests of their own. The wasps consumed 1.9 times more food per-capita, and showed a 12-fold increase in queen-turnovers and a fourfold increase in new nest initiations in the test nests as compared to the control nests. In other words, excess food made the wasps less cooperative and more selfish.
In the test nests, we fed every wasp as long as it accepted food. Thus, we had no control over the duration of feeding and therefore could not have sham controls. Feeding the test nests itself was extremely labour intensive and it was not possible for us to devote additional time for the sham controls. Moreover, the wasps usually become aggressive and attack the forceps if there is no food at the tip of it but accept food from the tip of the forceps without showing any aggressive behaviour. Nevertheless, we cannot, of course, entirely rule out some effect of the disturbance we may have caused while hand-feeding the wasps in the test colonies. To partly mitigate this problem, we constructed a separate linear model with food consumption as the predictor variable, without regard to control or test colony. In spite of considerable variation in food consumption within the control and test colonies, rates of queen turnover and new nest foundation increased significantly with food consumption. Besides, there is evidence in this and other species that extra food results in increased ovarian development (Gadagkar et al. 1988, 1991; Karsai and Hunt 2002; Shukla et al. 2013). All we can say is that we tried our best to cause as little disturbance as possible. Most of the time, the wasps did not even see the forceps but a morsel of food simply appeared close to the nest which they picked up. We routinely marked every wasp with two or more spots of colour paints. Such marking should cause much greater disturbance than the feeding and yet we have never seen any aggressive behaviour, queen turnover or leaving the nest, in response to the marking. Although we tried our best to minimise the disturbance and have reasons to suspect that there was no significant disturbance, we cannot entirely rule out the possibility that disturbance may have been one of the factors in accounting for our results. We will continue to explore the possibility of controlling for possible disturbance in future studies.
What might be the proximate and ultimate explanations for this phenomenon?
At the proximate level, adult nutrition is required for the development of ovaries (Molina and O’Donnell 2008a) and fat bodies (Hunt 1991), and for caste-specific gene expression (Berens et al., 2015), all of which facilitate reproduction. As also observed in another primitively eusocial wasp, Mischocyttarus mastigophorus (Molina and O’Donnell 2008a), nutrition led to increased expression of dominance behaviour. In yet another study with the primitively eusocial wasp, Polistes dominulus, supplemental food in addition to ad libitum food led to changes in facial patterns in workers, such that these workers signalled higher dominance status (Tibbetts and Curtis 2007). This may be relevant in R. marginata, where queen succession is accompanied by intense aggression. Similarly, a recent study in the primitively eusocial wasp, Mischocyttarus cerebrus, showed that the beta individual in a colony demonstrated high levels of dominance behaviour before replacing the alpha and such high dominance behaviour also led towards ovarian development in these new alpha individuals (da Silva et al. 2020). In R. marginata, we have evidence that aggression may be required for the potential queens to develop their ovaries rapidly (Lamba et al. 2007). Even more telling is our observation in a previous study that wasps form off-nest aggregations near the feeding stations where they engage in dominance-subordinate interaction and consume food before deserting their natal nests to initiate new nests (Brahma et al. 2019). Thus, the causal chain is likely to go from nutrition to aggression to ovary development and finally to queen-turnover or new nest foundation. Our results are in concurrence with the dominance-nutrition hypothesis that postulates that dominance in the colony, nutrition and reproductive physiology are linked in independent founding species like the primitively eusocial wasps (Markiewicz and O’Donnell 2001). Studies testing this hypothesis in Mischocyttarus mastigophorus showed that dominant individuals of the colony are nutritionally better-off than those which engage in energy-intensive tasks like foraging and the former end up developing their ovaries and nutritional storages (Molina and O’Donnell 2008b; O’Donnell et al. 2018; Fiocca et al. 2020b).
At the ultimate level, our results fulfil our expectation of more workers opting to gain direct fitness in the presence of excess nutrition. Queen turnovers and new nest foundations are normal phenomena that occur throughout the year in this tropical species with perennial indeterminate colony cycles. It is reasonable to expect that the wasps will be selected to continuously assess their chances of obtaining direct fitness either by replacing the queen of their colony and taking over as the next queen or by deserting their natal colony to initiate a successful new colony of their own. Their chances of becoming replacement queens are known to increase with their age (Bang and Gadagkar, 2012), and their chances of reverting to a solitary foundress role are known to decrease with their age and increase with nutrition (Brahma et al. 2018a). We also have evidence that the wasps are capable of choosing their nesting strategies based on their brood rearing abilities (Shakarad and Gadagkar 1997).
Nevertheless, it is intriguing that the wasps in the control nests did not consume more food from the feeding station and similarly increase their chances of future reproduction and direct fitness. One possible explanation for this is that in nature, foraging is likely to be a costly behaviour in terms of both energy expenditure and predation risk. Foraging is regulated by a decentralised, self-organised mechanism with the intranidal wasps conveying information about colony hunger levels to the foragers through dominance behaviour (Bruyndonckx et al. 2006; Lamba et al. 2008). It may therefore be maladaptive for the wasps in nature to make foraging trips unless prompted by the intranidal wasps. The wasps in our walk-in cages may be continuing to adopt strategies appropriate for their natural environment even though it may seem maladaptive in the new artificial environment. Consistent with this conjecture, we found that the wasps in the test colonies consumed much less food from the feeding station as compared to wasps from the control colonies. Be that as it may, our results suggest that cooperation is maintained in natural colonies, at least in part, by a form of nutritional castration. By releasing them from this nutritional constraint, we were able to make the wasps more selfish, giving us confidence that we correctly understand at least some of the factors that maintain social life in R. marginata.
Availability of data and material
Dataset is available in the supplementary material.
Code availability
All R-codes used for analysis are available in the supplementary material.
References
Andrade ACR, Miranda EA, Del Lama MA, Nascimento FS (2016) Reproductive concessions between related and unrelated members promote eusociality in bees. Sci Rep 6:26635. https://doi.org/10.1038/srep26635
Bang A, Gadagkar R (2012) Reproductive queue without overt conflict in the primitively eusocial wasp Ropalidia marginata. Proc Natl Acad Sci 109:14494–14499. https://doi.org/10.1073/pnas.1212698109
Berens AJ, Hunt JH, Toth AL (2015) Nourishment level affects caste-related gene expression in Polistes wasps. BMC Genomics 16:235. https://doi.org/10.1186/s12864-015-1410-y
Bhadra A, Gadagkar R (2008) We know that the wasps ‘know’: cryptic successors to the queen in Ropalidia marginata. Biol Lett 4:634–637. https://doi.org/10.1098/rsbl.2008.0455
Brahma A, Mandal S, Gadagkar R (2019) To leave or to stay: direct fitness through natural nest foundation in a primitively eusocial wasp. Insect Soc 66:335–342. https://doi.org/10.1007/s00040-019-00702-2
Brahma A, Mandal S, Gadagkar R (2018) Current indirect fitness and future direct fitness are not incompatible. Biol Lett 14:20170592. https://doi.org/10.1098/rsbl.2017.0592
Brahma A, Mandal S, Gadagkar R (2018) Emergence of cooperation and division of labor in the primitively eusocial wasp Ropalidia marginata. Proc Natl Acad Sci USA 115:756–761. https://doi.org/10.1073/pnas.1714006115
Bruyndonckx N, Kardile SP, Gadagkar R (2006) Dominance behaviour and regulation of foraging in the primitively eusocial wasp Ropalidia marginata (Lep.) (Hymenoptera: Vespidae). Behav Proc 72:100–103
da Silva RC, Prato A, Oi CA et al (2020) Dominance hierarchy, ovarian activity and cuticular dydrocarbons in the primitively eusocial wasp Mischocyttarus cerberus (Vespidae, Polistinae, Mischocyttarini). J Chem Ecol 46:835–844. https://doi.org/10.1007/s10886-020-01206-1
Field J, Cant MA (2009) Reproductive skew in primitively eusocial wasps: how useful are current models? Reproductive skew in vertebrates. Cambridge University Press, Cambridge, pp 773–780
Field J, Shreeves G, Sumner S (1999) Group size, queuing, and helping decisions in facultatively eusocial hover wasps. Behav EcolSociobiol 45:378–385
Fiocca K, Capobianco K, Fanwick E et al (2020) Reproductive physiology corresponds to adult nutrition and task performance in a Neotropical paper wasp: a test of dominance-nutrition hypothesis predictions. Behav Ecol Sociobiol 74:114. https://doi.org/10.1007/s00265-020-02898-x
Gadagkar R (2001) The Social Biology of Ropalidia marginata: Toward Understanding the Evolution of Eusociality. Harvard University Press, Cambridge, Massachusetts
Gadagkar R (2017) Choosing a New Queen: Consensus without conflict in a Social Wasp Colony. In: Landscapes of collectivity in the life sciences. The MIT Press, Cambridge, Massachusetts
Gadagkar R (2009) Interrogating an insect society. Proc Natl Acad Sci 106:10407–10414. https://doi.org/10.1073/pnas.0904317106
Gadagkar R, Bhagavan S, Chandrashekara K, Vinutha C (1991) The role of larval nutrition in pre-imaginal biasing of caste in the primitively eusocial wasp Ropalidia marginata (Hymenoptera: Vespidae). Ecol Entomol 16:435–440
Gadagkar R, Chandrashekara K, Chandran S, Bhagavan S (1993) Serial polygyny in the primitively eusocial wasp Ropalidia marginata: implications for the evolution of sociality. In: Queen number and sociality in insects. Oxford University Press, p 28
Gadagkar R, Joshi NV (1982) Behaviour of the Indian social wasp Ropalidia cyathiformis on a nest of separate combs (Hymenoptera: Vespidae). J Zool 198:27–37. https://doi.org/10.1111/j.1469-7998.1982.tb02058.x
Gadagkar R, Vinutha C, Shanubhogue A, Gore AP (1988) Pre-imaginal biasing of caste in a primitively eusocial insect. Proc R Soc Lond B 233:175–189. https://doi.org/10.1098/rspb.1988.0017
Gadau J, Fewell JE (2009) Organization of Insect Societies - From Genome to Sociocomplexity. Harvard University Press, Cambridge, Massachusetts
Heinze J (2004) Reproductive conflict in insect societies. Advances in the study of behaviour. Elsevier Academic Press, Cambridge, pp 1–55
Hunt JH (1991) Nourishment and the Evolution of the Social Vespidae. In: The Social Biology of Wasps. Cornell University Press
Karsai IN, Hunt JH (2002) Food quantity affects traits of offspring in the paper wasp Polistes metricus (Hymenoptera: Vespidae). Environ Entomol 31:8
Lamba S, Chandrasekhar K, Gadagkar R (2008) Signaling hunger through aggression - the regulation of foraging in a primitively eusocial wasp. Naturwissenschaften 95:677–680
Lamba S, Kazi YC, Deshpande S et al (2007) A possible novel function of dominance behaviour in queen-less colonies of the primitively eusocial wasp Ropalidia marginata. Behav Proc 74:351–356. https://doi.org/10.1016/j.beproc.2006.12.003
Leadbeater E, Carruthers JM, Green JP et al (2011) Nest inheritance is the missing source of direct fitness in a primitively eusocial insect. Science 333:874–876. https://doi.org/10.1126/science.1205140
Markiewicz D, O’Donnell S (2001) Social dominance, task performance and nutrition: implications for reproduction in eusocial wasps. J Comp Physiol A 187:327–333. https://doi.org/10.1007/s003590100204
Molina Y, O’Donnell S (2008) A developmental test of the dominance-nutrition hypothesis: linking adult feeding, aggression, and reproductive potential in the paperwasp Mischocyttarus mastigophorus. Ethol Ecol Evol 20:125–139. https://doi.org/10.1080/08927014.2008.9522533
Molina Y, O’Donnell S (2009) Worker reproductive competition affects division of labor in a primitively social paperwasp (Polistes instabilis). Insect Soc 56:14–20. https://doi.org/10.1007/s00040-008-1027-0
O’Donnell S, Fiocca K, Campbell M et al (2018) Adult nutrition and reproductive physiology: a stable isotope analysis in a eusocial paper wasp (Mischocyttarus mastigophorus, Hymenoptera: Vespidae). Behav Ecol Sociobiol 72:86. https://doi.org/10.1007/s00265-018-2501-y
Queller DC, Zacchi F, Cervo R et al (2000) Unrelated helpers in a social insect. Nature 405:784–787. https://doi.org/10.1038/35015552
Ross KG, Matthews RW (eds) (1991) The Social biology of wasps. Cornell University Press, Ithaca
Shakarad M, Gadagkar R (1997) Do social wasps choose nesting strategies based on their brood rearing abilities? Naturwissenschaften 84:79–82
Shukla S, Chandran S, Gadagkar R (2013) Ovarian developmental variation in the primitively eusocial wasp Ropalidia marginata suggests a gateway to worker ontogeny and the evolution of sociality. J Exp Biol 216:181–187. https://doi.org/10.1242/jeb.073148
Southon RJ, Radford AN, Sumner S (2020) High reproductive skew in the Neotropical paper wasp Polistes lanio. Insect Soc 67:451–456. https://doi.org/10.1007/s00040-020-00780-7
Strassmann JE, Meyer DC (1983) Gerontocracy in the social wasp, Polistes exclamans. Anim Behav 31:431–438
Sumner S, Kelstrup H, Fanelli D (2010) Reproductive constraints, direct fitness and indirect fitness benefits explain helping behaviour in the primitively eusocial wasp, Polistes canadensis. Proc R Soc B 277:1721–1728. https://doi.org/10.1098/rspb.2009.2289
Taylor BA, Cini A, Cervo R et al (2020) Queen succession conflict in the paper wasp Polistes dominula is mitigated by age-based convention. Behav Ecol 31:992–1002. https://doi.org/10.1093/beheco/araa045
Tibbetts EA, Curtis TR (2007) Rearing conditions influence quality signals but not individual identity signals in Polistes wasps. Behav Ecol 18:602–607. https://doi.org/10.1093/beheco/arm013
Tsuchida K, Saigo T, Asai K et al (2020) Reproductive workers insufficiently signal their reproductive ability in a paper wasp. Behav Ecol 31:577–590. https://doi.org/10.1093/beheco/arz212
Tsuchida K, Saigo T, Nagata N et al (2003) Queen-worker conflicts over male production and sex allocation in a primitively eusocial wasp. Evolution 57:2365–2373. https://doi.org/10.1111/j.0014-3820.2003.tb00248.x
Unnikrishnan S, Gadagkar R (2017) Dominance based reproductive queue in the primitively eusocial wasp, Ropalidia cyathiformis. Insect Soc 64:495–503. https://doi.org/10.1007/s00040-017-0568-5
Wilson EO (1971) The insect societies. Belknap Press of Harvard University Press, Cambridge, Massachusetts
Funding
Supported by grants from the Department of Science and Technology (EMR/2016/000658 and SR/S9/YOCP-35/2016), Department of Biotechnology and Council of Scientific and Industrial Research (37 (1691)/17/EMR-II), Government of India (to RG), the UGC-D.S. Kothari post-doctoral fellowship (No.F.4–2/2006 (BSR)/BL/17–18/0183) (JUK) and the IISc-RA fellowship (AB).
Author information
Authors and Affiliations
Contributions
AB and RG: conceived study, AB, JUK and RG: designed study, AB, JUK and SKC: conducted study, AB and JUK: analysed data, and AB, JUK and RG: co-wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krishnan, J.U., Brahma, A., Chavan, S.K. et al. Nutrition induced direct fitness for workers in a primitively eusocial wasp. Insect. Soc. 68, 319–325 (2021). https://doi.org/10.1007/s00040-021-00835-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-021-00835-3