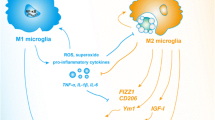
Abstract
Microglia are resident immune cells in the brain and play a central role in the development and surveillance of the nervous system. Extensive gliosis is a common pathological feature of several neurodegenerative diseases, such as Alzheimer's disease (AD), the most common cause of dementia. Microglia can respond to multiple inflammatory insults and later transform into different phenotypes, such as pro- and anti-inflammatory phenotypes, thereby exerting different functions. In recent years, an increasing number of studies based on both traditional bulk sequencing and novel single-cell/nuclear sequencing and multi-omics analysis, have shown that microglial phenotypes are highly heterogeneous and dynamic, depending on the severity and stage of the disease as well as the particular inflammatory milieu. Thus, redirecting microglial activation to beneficial and neuroprotective phenotypes promises to halt the progression of neurodegenerative diseases. To this end, an increasing number of studies have focused on unraveling heterogeneous microglial phenotypes and their underlying molecular mechanisms, including those due to epigenetic and non-coding RNA modulations. In this review, we summarize the epigenetic mechanisms in the form of DNA and histone modifications, as well as the general non-coding RNA regulations that modulate microglial activation during immunopathogenesis of neurodegenerative diseases and discuss promising research approaches in the microglial era.





Similar content being viewed by others
Availability of data and materials
Not applicable.
Abbreviations
- 5-azaC:
-
5-Azacytidine
- 5-caC:
-
5-Carboxylcytosine
- 5-fC:
-
5-Formylcytosine
- 5-hmC:
-
5-Hydroxymethylcytosine
- 5-mC:
-
5-Methylcytosine
- AAV:
-
Adeno-associated virus
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- ARG1:
-
Arginase 1
- Aβ:
-
β-Amyloid peptide
- BBB:
-
Blood–brain barrier
- BDNF:
-
Brain-derived neurotrophic factor
- BET:
-
Bromodomain and extraterminal domain
- circRNAs:
-
Circular RNAs
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- DA:
-
Dopaminergic
- DAM:
-
Disease-associated microglia
- DAMPs:
-
Damage-associated molecular patterns
- DHEA:
-
Dehydroepiandrosterone
- DNMTs:
-
DNA methyltransferases
- EAE:
-
Experimental autoimmune encephalomyelitis
- EVs:
-
Extracellular vesicles
- EZH2:
-
Zeste homolog-2
- FTO:
-
Fat mass and obesity-associated protein
- GF:
-
Germ-free
- GWAS:
-
Genome-wide association studies
- H3K27:
-
Histone H3 at lysine 27
- H3S10phK14ac:
-
Phospho(S10)-acetylation(K14) of histone H3
- HD:
-
Huntington’s disease
- HDACs:
-
Histone deacetylases
- HMC3:
-
Human Microglia Clone 3
- HMT:
-
Histone methyltransferase
- hPSCs:
-
Human pluripotent stem cells
- iMGs:
-
IPSCs-derived microglia
- iPSCs:
-
Induced pluripotent stem cells
- JMJD3:
-
Jumonji domain-containing 3
- LDAM:
-
Lipid droplet-accumulating microglia
- lncRNAs:
-
Long non-coding RNAs
- Lnc-SNHG1:
-
LncRNA small nucleolar RNA host gene 1
- LOAD:
-
Late-onset Alzheimer’s disease
- m6A:
-
N6-methyladenosine
- m6Am:
-
N6,2′-O-dimethyladenosine
- MeCP2:
-
Methyl-CpG binding protein 2
- METTL3:
-
Methyltransferase-like protein 3
- MGnD:
-
Microglial neurodegenerative phenotype
- MHC-II:
-
Major histocompatibility complex II
- mHTT:
-
Mutant huntingtin
- miRNAs:
-
MicroRNAs
- MS:
-
Multiple sclerosis
- NAMPs:
-
Neurodegeneration-associated molecular patterns
- NF-κB:
-
Nuclear factor kappa-B
- NRF2:
-
Nuclear factor erythroid 2-related factor 2
- OXPHOS:
-
Oxidative phosphorylation
- PAM:
-
Plaque-associated microglia
- PAMPs:
-
Pathogen-associated molecular patterns
- PD:
-
Parkinson's disease
- PET:
-
Positron emission tomography
- PRC2:
-
Polycomb repressive complex-2
- PSCs:
-
Pluripotent stem cells
- RACK1:
-
Receptor for activated c kinase 1
- RBPs:
-
RNA binding proteins
- rmTBI:
-
Repetitive mild TBI
- ROS:
-
Reactive oxygen species
- SAH:
-
S-Adenosylhomocysteine
- SIRT1:
-
Sirtuin 1
- SN:
-
Substantia nigra
- SNHG1:
-
Small nucleolar RNA host gene 1
- SOCS-1:
-
Suppressor of cytokine signaling 1
- SPF:
-
Specific-pathogen-free
- TBI:
-
Traumatic brain injury
- TDP43:
-
TAR DNA-binding protein 43
- TEM:
-
Transmission electron microscopy
- TET:
-
Ten-eleven translocation
- TREM2:
-
Triggering receptor expressed on myeloid cells 2
- TSA:
-
Trichostatin A
- TSPO:
-
18 KDa Translocator protein
- TSS:
-
Transcriptional start sites
- VPA:
-
Valproic acid
- WAM:
-
White matter-associated microglia
References
Lane CA, Hardy J, Schott JM (2018) Alzheimer’s disease. Eur J Neurol 25(1):59–70. https://doi.org/10.1111/ene.13439
Poewe W et al (2017) Parkinson disease. Nat Rev Dis Primers 3:17013. https://doi.org/10.1038/nrdp.2017.13
Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 15(12):1257–1272. https://doi.org/10.1016/S1474-4422(16)30230-7
Selkoe DJ (2012) Preventing Alzheimer’s disease. Science 337(6101):1488–1492. https://doi.org/10.1126/science.1228541
Zaletel I et al (2018) Early impairments of hippocampal neurogenesis in 5xFAD mouse model of Alzheimer’s disease are associated with altered expression of SOXB transcription factors. J Alzheimers Dis 65(3):963–976. https://doi.org/10.3233/JAD-180277
Fahnestock M, Shekari A (2019) ProNGF and neurodegeneration in Alzheimer’s disease. Front Neurosci 13:129. https://doi.org/10.3389/fnins.2019.00129
Wightman DP et al (2020) Largest GWAS (N=1,126,563) of Alzheimer’s disease implicates microglia and immune cells. medRxiv. https://doi.org/10.1101/2020.11.20.20235275
Seshadri S et al (2010) Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA 303(18):1832–1840. https://doi.org/10.1001/jama.2010.574
Morabito S et al (2021) Single-nucleus chromatin accessibility and transcriptomic characterization of Alzheimer’s disease. Nat Genet 53(8):1143–1155. https://doi.org/10.1038/s41588-021-00894-z
Prinz M, Jung S, Priller J (2019) Microglia biology: one century of evolving concepts. Cell 179(2):292–311. https://doi.org/10.1016/j.cell.2019.08.053
von Bartheld CS, Bahney J, Herculano-Houzel S (2016) The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol 524(18):3865–3895. https://doi.org/10.1002/cne.24040
Sierra A, Paolicelli RC, Kettenmann H (2019) Cien Anos de Microglia: milestones in a century of microglial research. Trends Neurosci 42(11):778–792. https://doi.org/10.1016/j.tins.2019.09.004
Le W, Wu J, Tang Y (2016) Protective microglia and their regulation in Parkinson’s disease. Front Mol Neurosci 9:89. https://doi.org/10.3389/fnmol.2016.00089
Zhou N et al (2019) Transcriptional mechanism of IRF8 and PU.1 governs microglial activation in neurodegenerative condition. Protein Cell 10(2):87–103. https://doi.org/10.1007/s13238-018-0599-3
Gu X et al (2013) Oxidative stress induces DNA demethylation and histone acetylation in SH-SY5Y cells: potential epigenetic mechanisms in gene transcription in Aβ production. Neurobiol Aging 34(4):1069–1079. https://doi.org/10.1016/j.neurobiolaging.2012.10.013
Ayata P et al (2018) Epigenetic regulation of brain region-specific microglia clearance activity. Nat Neurosci 21(8):1049–1060. https://doi.org/10.1038/s41593-018-0192-3
Thangaraj A et al (2020) Targeting endoplasmic reticulum stress and autophagy as therapeutic approaches for neurological diseases. Int Rev Cell Mol Biol 350:285–325. https://doi.org/10.1016/bs.ircmb.2019.11.001
Zotova E et al (2013) Inflammatory components in human Alzheimer’s disease and after active amyloid-beta42 immunization. Brain 136(Pt 9):2677–2696. https://doi.org/10.1093/brain/awt210
Singh-Bains MK et al (2019) Altered microglia and neurovasculature in the Alzheimer’s disease cerebellum. Neurobiol Dis 132:104589. https://doi.org/10.1016/j.nbd.2019.104589
Pavese N et al (2006) Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology 66(11):1638–1643. https://doi.org/10.1212/01.wnl.0000222734.56412.17
Sapp E et al (2001) Early and progressive accumulation of reactive microglia in the Huntington disease brain. J Neuropathol Exp Neurol 60(2):161–172. https://doi.org/10.1093/jnen/60.2.161
Cosenza-Nashat M et al (2009) Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol 35(3):306–328. https://doi.org/10.1111/j.1365-2990.2008.01006.x
Venneti S et al (2008) The positron emission tomography ligand DAA1106 binds with high affinity to activated microglia in human neurological disorders. J Neuropathol Exp Neurol 67(10):1001–1010. https://doi.org/10.1097/NEN.0b013e318188b204
Zhang L et al (2021) Recent developments on PET radiotracers for TSPO and their applications in neuroimaging. Acta Pharm Sin B 11(2):373–393. https://doi.org/10.1016/j.apsb.2020.08.006
Pascoal TA et al (2021) Microglial activation and tau propagate jointly across Braak stages. Nat Med 27(9):1592–1599. https://doi.org/10.1038/s41591-021-01456-w
Joers V et al (2020) Microglia, inflammation and gut microbiota responses in a progressive monkey model of Parkinson’s disease: a case series. Neurobiol Dis 144:105027. https://doi.org/10.1016/j.nbd.2020.105027
Deczkowska A et al (2018) Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell 173(5):1073–1081. https://doi.org/10.1016/j.cell.2018.05.003
Subhramanyam CS et al (2019) Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol 94:112–120. https://doi.org/10.1016/j.semcdb.2019.05.004
Cui W et al (2020) Inhibition of TLR4 induces M2 microglial polarization and provides neuroprotection via the NLRP3 inflammasome in Alzheimer’s disease. Front Neurosci 14:444. https://doi.org/10.3389/fnins.2020.00444
Sorrentino S et al (2021) Microglial heterogeneity and its potential role in driving phenotypic diversity of Alzheimer’s disease. Int J Mol Sci. https://doi.org/10.3390/ijms22052780
Eikelenboom P et al (2012) Whether, when and how chronic inflammation increases the risk of developing late-onset Alzheimer’s disease. Alzheimers Res Ther 4(3):15. https://doi.org/10.1186/alzrt118
Blandini F (2013) Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J Neuroimmune Pharm 8(1):189–201. https://doi.org/10.1007/s11481-013-9435-y
Agostinho P, Cunha RA, Oliveira C (2010) Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr Pharm Design 16(25):2766–2778
Association AS (2019) 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 15(3):321–387. https://doi.org/10.1016/j.jalz.2019.01.010
Gao HM et al (2008) Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci 28(30):7687–7698. https://doi.org/10.1523/JNEUROSCI.0143-07.2008
Benraiss A et al (2016) Human glia can both induce and rescue aspects of disease phenotype in Huntington disease. Nat Commun 7:11758. https://doi.org/10.1038/ncomms11758
Halle A et al (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat Immunol 9(8):857–865. https://doi.org/10.1038/ni.1636
Neumann M et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314(5796):130–133. https://doi.org/10.1126/science.1134108
Valekova I et al (2016) Revelation of the IFNalpha, IL-10, IL-8 and IL-1beta as promising biomarkers reflecting immuno-pathological mechanisms in porcine Huntington’s disease model. J Neuroimmunol 293:71–81. https://doi.org/10.1016/j.jneuroim.2016.02.012
Colonna M, Butovsky O (2017) Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 35:441–468. https://doi.org/10.1146/annurev-immunol-051116-052358
Greenhalgh AD, David S, Bennett FC (2020) Immune cell regulation of glia during CNS injury and disease. Nat Rev Neurosci 21(3):139–152. https://doi.org/10.1038/s41583-020-0263-9
Bartels T, De Schepper S, Hong S (2020) Microglia modulate neurodegeneration in Alzheimer’s and Parkinson’s diseases. Science 370(6512):66–69. https://doi.org/10.1126/science.abb8587
Itagaki S et al (1989) Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol 24(3):173–182. https://doi.org/10.1016/0165-5728(89)90115-x
Yin, et al (2017) Immune hyperreactivity of Abeta plaque-associated microglia in Alzheimer’s disease. Neurobiol Aging 55:115–122. https://doi.org/10.1016/j.neurobiolaging.2017.03.021
Condello C et al (2015) Microglia constitute a barrier that prevents neurotoxic protofibrillar Abeta42 hotspots around plaques. Nat Commun 6:6176. https://doi.org/10.1038/ncomms7176
Marschallinger J et al (2020) Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci 23(2):194–208. https://doi.org/10.1038/s41593-019-0566-1
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53(2):1181–1194. https://doi.org/10.1007/s12035-014-9070-5
Song WM, Colonna M (2018) The identity and function of microglia in neurodegeneration. Nat Immunol 19(10):1048–1058. https://doi.org/10.1038/s41590-018-0212-1
Pena-Altamira E et al (2017) Nutritional and pharmacological strategies to regulate microglial polarization in cognitive aging and Alzheimer’s disease. Front Aging Neurosci 9:175. https://doi.org/10.3389/fnagi.2017.00175
Businaro R et al (2018) Modulation of inflammation as a way of delaying Alzheimer’s disease progression: the diet’s role. Curr Alzheimer Res 15(4):363–380. https://doi.org/10.2174/1567205014666170829100100
Yang Z et al (2019) Platycodigenin as potential drug candidate for Alzheimer’s disease via modulating microglial polarization and neurite regeneration. Molecules (Basel, Switzerland). https://doi.org/10.3390/molecules24183207
Tarassishin L, Suh H-S, Lee SC (2014) LPS and IL-1 differentially activate mouse and human astrocytes: role of CD14. Glia. https://doi.org/10.1002/glia.22657
Ji J et al (2019) The intra-nuclear SphK2-S1P axis facilitates M1-to-M2 shift of microglia via suppressing HDAC1-mediated KLF4 deacetylation. Front Immunol 10:1241. https://doi.org/10.3389/fimmu.2019.01241
He Y et al (2020) IL-4 switches microglia/macrophage M1/M2 polarization and alleviates neurological damage by modulating the JAK1/STAT6 pathway following ICH. Neuroscience 437:161–171. https://doi.org/10.1016/j.neuroscience.2020.03.008
Yu Z et al (2015) MSX3 switches microglia polarization and protects from inflammation-induced demyelination. J Neurosci 35(16):6350–6365. https://doi.org/10.1523/JNEUROSCI.2468-14.2015
Li Y et al (2021) Ultrasound controlled anti-inflammatory polarization of platelet decorated microglia for targeted ischemic stroke therapy. Angew Chem Int Ed Engl 60(10):5083–5090. https://doi.org/10.1002/anie.202010391
Zeng F et al (2018) Custom-made ceria nanoparticles show a neuroprotective effect by modulating phenotypic polarization of the microglia. Angew Chem Int Ed Engl 57(20):5808–5812. https://doi.org/10.1002/anie.201802309
Dumas AA, Borst K, Prinz M (2021) Current tools to interrogate microglial biology. Neuron 109(18):2805–2819. https://doi.org/10.1016/j.neuron.2021.07.004
Paolicelli RaS, Stevens A, Tremblay B, Aguzzi M-E, Ajami A, Amit B, Audinat I, Bechmann E, Bennett I, Bennett M, Bessis F, Biber A, Bilbo K, Blurton-Jones S, Boddeke M, Brites E, Brône D, Brown B, Butovsky GC, Carson O, Castellano MJ, Colonna B, Cowley M, Cunningham SA, Davalos C, De Jager D, De Strooper PL, Dénes B, Eggen A, Eyo BJL, Galea U, Garel E, Ginhoux S, Glass F, Gokce CK, Gomez-Nicola O, González D, Gordon B, Graeber S, Greenhalgh MB, Gressens AD, Greter P, Gutmann M, Haass DH, Heneka C, Heppner MT, Hong F, Jung S, Kettenmann S, Kipnis H, Koyama J, Lemke R, Lynch G, Majewska M, Malcangio A, Malm M, Mancuso T, Matteoli R, McColl M, Miron N, Molofsky VE, Monje AV, Mracsko M, Nadjar E, Neher A, Neniskyte JJ, Neumann U, Noda H, Peng M, Peri B, Perry F, Popovich HV, Priller PG, Ragozzino J, Ransohoff D, Salter RM, Schaefer MW, Schafer A, Schwartz DP, Simons M, Streit M, Tay WJ, Tsai TL, Verkhratsky L-H, von Bernhardi A, Wake R, Wittamer H, Wolf V, Wu SA, Wyss-Coray L-JT, (2022) Defining microglial states and nomenclature: a roadmap to 2030. https://doi.org/10.2139/ssrn.4065080
Stratoulias V et al (2019) Microglial subtypes: diversity within the microglial community. EMBO J 38(17):e101997. https://doi.org/10.15252/embj.2019101997
Schwabenland M et al (2021) Analyzing microglial phenotypes across neuropathologies: a practical guide. Acta Neuropathol 142(6):923–936. https://doi.org/10.1007/s00401-021-02370-8
Sankowski R et al (2019) Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat Neurosci 22(12):2098–2110. https://doi.org/10.1038/s41593-019-0532-y
Li Y et al (2022) Decoding the temporal and regional specification of microglia in the developing human brain. Cell Stem Cell. https://doi.org/10.1016/j.stem.2022.02.004
Bottcher C et al (2019) Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat Neurosci 22(1):78–90. https://doi.org/10.1038/s41593-018-0290-2
Uriarte Huarte O et al (2021) Single-cell transcriptomics and in situ morphological analyses reveal microglia heterogeneity across the nigrostriatal pathway. Front Immunol 12:639613. https://doi.org/10.3389/fimmu.2021.639613
Abellanas MA et al (2019) Midbrain microglia mediate a specific immunosuppressive response under inflammatory conditions. J Neuroinflamm 16(1):233. https://doi.org/10.1186/s12974-019-1628-8
Mathys H et al (2017) Temporal tracking of microglia activation in neurodegeneration at single-cell resolution. Cell Rep 21(2):366–380. https://doi.org/10.1016/j.celrep.2017.09.039
Keren-Shaul H et al (2017) A Unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169(7):1276-1290 e17. https://doi.org/10.1016/j.cell.2017.05.018
Wang Y et al (2015) TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160(6):1061–1071. https://doi.org/10.1016/j.cell.2015.01.049
Yerbury JJ et al (2016) Walking the tightrope: proteostasis and neurodegenerative disease. J Neurochem 137(4):489–505. https://doi.org/10.1111/jnc.13575
Singh N et al (2022) BACE-1 inhibition facilitates the transition from homeostatic microglia to DAM-1. Sci Adv 8(24):eabo1286. https://doi.org/10.1126/sciadv.abo1286
Ulrich JD et al (2014) Altered microglial response to Abeta plaques in APPPS1-21 mice heterozygous for TREM2. Mol Neurodegener 9:20. https://doi.org/10.1186/1750-1326-9-20
McQuade A et al (2020) Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer’s disease. Nat Commun 11(1):5370. https://doi.org/10.1038/s41467-020-19227-5
Leyns CEG et al (2017) TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc Natl Acad Sci U S A 114(43):11524–11529. https://doi.org/10.1073/pnas.1710311114
Krasemann S et al (2017) The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47(3):566-581 e9. https://doi.org/10.1016/j.immuni.2017.08.008
Safaiyan S et al (2021) White matter aging drives microglial diversity. Neuron 109(7):1100-1117 e10. https://doi.org/10.1016/j.neuron.2021.01.027
Toledo JB et al (2014) CSF Apo-E levels associate with cognitive decline and MRI changes. Acta Neuropathol 127(5):621–632. https://doi.org/10.1007/s00401-013-1236-0
Lopes KP et al (2022) Genetic analysis of the human microglial transcriptome across brain regions, aging and disease pathologies. Nat Genet 54(1):4–17. https://doi.org/10.1038/s41588-021-00976-y
Coppieters N, Dragunow M (2011) Epigenetics in Alzheimer’s disease: a focus on DNA modifications. Curr Pharm Des 17(31):3398–3412. https://doi.org/10.2174/138161211798072544
Cho SH et al (2015) SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1beta. J Neurosci 35(2):807–818. https://doi.org/10.1523/JNEUROSCI.2939-14.2015
Matt MS, Lawson MA, Johnson RW (2016) Aging and peripheral lipopolysaccharide can modulate epigenetic regulators and decrease IL-1β promoter DNA methylation in microglia. Neurobiol Aging 47:1–9. https://doi.org/10.1016/j.neurobiolaging.2016.07.006
Matt SM et al (2018) Inhibition of DNA methylation with zebularine alters lipopolysaccharide-induced sickness behavior and neuroinflammation in mice. Front Neurosci 12:636. https://doi.org/10.3389/fnins.2018.00636
Moore LD, Le T, Fan G (2013) DNA methylation and its basic function. Neuropsychopharmacol 38(1):23–38. https://doi.org/10.1038/npp.2012.112
Lin HC et al (2009) S-Adenosylhomocysteine increases beta-amyloid formation in BV-2 microglial cells by increased expressions of beta-amyloid precursor protein and presenilin 1 and by hypomethylation of these gene promoters. Neurotoxicology 30(4):622–627. https://doi.org/10.1016/j.neuro.2009.03.011
Efimova OA et al (2020) Environmental epigenetics and genome flexibility: focus on 5-hydroxymethylcytosine. Int J Mol Sci. https://doi.org/10.3390/ijms21093223
Zhao J et al (2017) A genome-wide profiling of brain DNA hydroxymethylation in Alzheimer’s disease. Alzheimers Dement 13(6):674–688. https://doi.org/10.1016/j.jalz.2016.10.004
Khare T et al (2012) 5-hmC in the brain is abundant in synaptic genes and shows differences at the exon-intron boundary. Nat Struct Mol Biol 19(10):1037–1043. https://doi.org/10.1038/nsmb.2372
Szulwach KE et al (2011) 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat Neurosci 14(12):1607–1616. https://doi.org/10.1038/nn.2959
Hahn MA et al (2013) Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis. Cell Rep 3(2):291–300. https://doi.org/10.1016/j.celrep.2013.01.011
Zhang Y et al (2020) Selective loss of 5hmC promotes neurodegeneration in the mouse model of Alzheimer’s disease. FASEB J 34(12):16364–16382. https://doi.org/10.1096/fj.202001271R
Li L et al (2020) Reduction of Tet2 exacerbates early stage Alzheimer’s pathology and cognitive impairments in 2×Tg-AD mice. Hum Mol Genet 29(11):1833–1852. https://doi.org/10.1093/hmg/ddz282
Celarain N et al (2016) TREM2 upregulation correlates with 5-hydroxymethycytosine enrichment in Alzheimer’s disease hippocampus. Clin Epigenet 8:37. https://doi.org/10.1186/s13148-016-0202-9
Wu X, Zhang Y (2017) TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet 18(9):517–534. https://doi.org/10.1038/nrg.2017.33
He X-B et al (2015) Vitamin C facilitates dopamine neuron differentiation in fetal midbrain through TET1- and JMJD3-dependent epigenetic control manner. Stem Cells 33(4):1320–1332. https://doi.org/10.1002/stem.1932
Zhang, et al (2013) Tet1 regulates adult hippocampal neurogenesis and cognition. Cell Stem Cell 13(2):237–245. https://doi.org/10.1016/j.stem.2013.05.006
Cochran JN et al (2020) Non-coding and loss-of-function coding variants in TET2 are associated with multiple neurodegenerative diseases. Am J Hum Genet 106(5):632–645. https://doi.org/10.1016/j.ajhg.2020.03.010
Cull AH et al (2017) Tet2 restrains inflammatory gene expression in macrophages. Exp Hematol 55:56-70 e13. https://doi.org/10.1016/j.exphem.2017.08.001
Carrillo-Jimenez A et al (2019) TET2 regulates the neuroinflammatory response in microglia. Cell Rep 29(3):697-713 e8. https://doi.org/10.1016/j.celrep.2019.09.013
Stewart-Morgan KR, Petryk N, Groth A (2020) Chromatin replication and epigenetic cell memory. Nat Cell Biol 22(4):361–371. https://doi.org/10.1038/s41556-020-0487-y
Bannister AJ, Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Res 21(3):381–395. https://doi.org/10.1038/cr.2011.22
Jambhekar A, Dhall A, Shi Y (2019) Roles and regulation of histone methylation in animal development. Nat Rev Mol Cell Biol 20(10):625–641. https://doi.org/10.1038/s41580-019-0151-1
Sun D et al (2017) LncRNA GAS5 inhibits microglial M2 polarization and exacerbates demyelination. EMBO Rep 18(10):1801–1816. https://doi.org/10.15252/embr.201643668
Margueron R, Reinberg D (2011) The Polycomb complex PRC2 and its mark in life. Nature 469(7330):343–349. https://doi.org/10.1038/nature09784
Xiang Y et al (2007) JMJD3 is a histone H3K27 demethylase. Cell Res 17(10):850–857. https://doi.org/10.1038/cr.2007.83
Tang Y et al (2014) Jmjd3 is essential for the epigenetic modulation of microglia phenotypes in the immune pathogenesis of Parkinson’s disease. Cell Death Differ 21(3):369–380. https://doi.org/10.1038/cdd.2013.159
Alexaki VI et al (2018) DHEA inhibits acute microglia-mediated inflammation through activation of the TrkA-Akt1/2-CREB-Jmjd3 pathway. Mol Psychiatr 23(6):1410–1420. https://doi.org/10.1038/mp.2017.167
Datta M et al (2018) Histone deacetylases 1 and 2 regulate microglia function during development, homeostasis, and neurodegeneration in a context-dependent manner. Immunity 48(3):514-529 e6. https://doi.org/10.1016/j.immuni.2018.02.016
Sun XY et al (2019) HDAC2 hyperexpression alters hippocampal neuronal transcription and microglial activity in neuroinflammation-induced cognitive dysfunction. J Neuroinflamm 16(1):249. https://doi.org/10.1186/s12974-019-1640-z
Kannan V et al (2013) Histone deacetylase inhibitors suppress immune activation in primary mouse microglia. J Neurosci Res 91(9):1133–1142. https://doi.org/10.1002/jnr.23221
Jiao F-Z et al (2018) Histone deacetylase 2 inhibitor CAY10683 alleviates lipopolysaccharide induced neuroinflammation through attenuating TLR4/NF-κB signaling pathway. Neurochem Res 43(6):1161–1170. https://doi.org/10.1007/s11064-018-2532-9
Xia M et al (2017) Proteomic analysis of HDAC3 selective inhibitor in the regulation of inflammatory response of primary microglia. Neural Plast. https://doi.org/10.1155/2017/6237351
Kim T et al (2019) HDAC inhibition by valproic acid induces neuroprotection and improvement of PD-like behaviors in LRRK2 R1441G transgenic mice. Exp Neurobiol 28(4):504–515. https://doi.org/10.5607/en.2019.28.4.504
Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9(11):798–809. https://doi.org/10.1038/nrc2734
Logotheti S, Putzer BM (2019) STAT3 and STAT5 targeting for simultaneous management of melanoma and autoimmune diseases. Cancers (Basel). https://doi.org/10.3390/cancers11101448
Zhu X et al (2017) HDAC3 negatively regulates spatial memory in a mouse model of Alzheimer’s disease. Aging Cell 16(5):1073–1082. https://doi.org/10.1111/acel.12642
Lin FL et al (2019) HADC8 Inhibitor WK2–16 therapeutically targets lipopolysaccharide-induced mouse model of neuroinflammation and microglial activation. Int J Mol Sci. https://doi.org/10.3390/ijms20020410
Hsing CH et al (2015) Histone deacetylase inhibitor trichostatin A ameliorated endotoxin-induced neuroinflammation and cognitive dysfunction. Mediators Inflamm. https://doi.org/10.1155/2015/163140
Su Q et al (2021) Trichostatin A ameliorates Alzheimer’s disease-related pathology and cognitive deficits by increasing albumin expression and Abeta clearance in APP/PS1 mice. Alzheimers Res Ther 13(1):7. https://doi.org/10.1186/s13195-020-00746-8
Jung KH et al (2015) RNA sequencing reveals distinct mechanisms underlying BET inhibitor JQ1-mediated modulation of the LPS-induced activation of BV-2 microglial cells. J Neuroinflamm 12:36. https://doi.org/10.1186/s12974-015-0260-5
Rigillo G et al (2018) LPS-induced histone H3 phospho(Ser10)-acetylation(Lys14) regulates neuronal and microglial neuroinflammatory response. Brain Behav Immun 74:277–290. https://doi.org/10.1016/j.bbi.2018.09.019
Pan RY et al (2022) Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab 34(4):634-648 e6. https://doi.org/10.1016/j.cmet.2022.02.013
Roundtree IA et al (2017) Dynamic RNA modifications in gene expression regulation. Cell 169(7):1187–1200. https://doi.org/10.1016/j.cell.2017.05.045
Shafik AM et al (2021) N6-methyladenosine dynamics in neurodevelopment and aging, and its potential role in Alzheimer’s disease. Genome Biol 22(1):17. https://doi.org/10.1186/s13059-020-02249-z
Han M et al (2020) Abnormality of m6A mRNA methylation is involved in Alzheimer’s disease. Front Neurosci 14:98. https://doi.org/10.3389/fnins.2020.00098
Yao Y et al (2018) Pterostilbene and 4’-methoxyresveratrol inhibited lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages. Molecules. https://doi.org/10.3390/molecules23051148
Szabo M, Gulya K (2013) Development of the microglial phenotype in culture. Neuroscience 241:280–295. https://doi.org/10.1016/j.neuroscience.2013.03.033
Martinez FO, Helming L, Gordon S (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27:451–483. https://doi.org/10.1146/annurev.immunol.021908.132532
Hsieh S-W et al (2020) M2b macrophage subset decrement as an indicator of cognitive function in Alzheimer’s disease. Psychiat Clin Neuros 74(7):383–391. https://doi.org/10.1111/pcn.13000
Famenini S et al (2017) Increased intermediate M1–M2 macrophage polarization and improved cognition in mild cognitive impairment patients on ω-3 supplementation. FASEB J 31(1):148–160. https://doi.org/10.1096/fj.201600677RR
Liu Y et al (2019) The -methyladenosine (mA)-forming enzyme METTL3 facilitates M1 macrophage polarization through the methylation of mRNA. Am J Physiol-Cell Ph 317(4):C762–C775. https://doi.org/10.1152/ajpcell.00212.2019
Gu X et al (2020) N6-methyladenosine demethylase FTO promotes M1 and M2 macrophage activation. Cell Signal 69:109553. https://doi.org/10.1016/j.cellsig.2020.109553
Ambros V (2004) The functions of animal microRNAs. Nature 431(7006):350–355. https://doi.org/10.1038/nature02871
Brettschneider J et al (2015) Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci 16(2):109–120. https://doi.org/10.1038/nrn3887
Gaudet AD et al (2018) MicroRNAs: roles in regulating neuroinflammation. Neuroscientist 24(3):221–245. https://doi.org/10.1177/1073858417721150
Karthikeyan A et al (2016) MicroRNAs: key players in microglia and astrocyte mediated inflammation in CNS pathologies. Curr Med Chem 23(30):3528–3546. https://doi.org/10.2174/0929867323666160814001040
Ponomarev ED et al (2011) MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat Med 17(1):64–70. https://doi.org/10.1038/nm.2266
Yao L et al (2018) MicroRNA-124 regulates the expression of MEKK3 in the inflammatory pathogenesis of Parkinson’s disease. J Neuroinflamm 15(1):13. https://doi.org/10.1186/s12974-018-1053-4
Yao L et al (2019) MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson’s disease. FASEB J 33(7):8648–8665. https://doi.org/10.1096/fj.201900363R
Pinto S et al (2017) Exosomes from NSC-34 Cells transfected with hSOD1-G93A are enriched in miR-124 and drive alterations in microglia phenotype. Front Neurosci 11:273. https://doi.org/10.3389/fnins.2017.00273
Edwards G 3rd, Moreno-Gonzalez I, Soto C (2017) Amyloid-beta and tau pathology following repetitive mild traumatic brain injury. Biochem Biophys Res Commun 483(4):1137–1142. https://doi.org/10.1016/j.bbrc.2016.07.123
Huang S et al (2018) Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J 32(1):512–528. https://doi.org/10.1096/fj.201700673R
Ge X et al (2020) Increased microglial exosomal miR-124-3p alleviates neurodegeneration and improves cognitive outcome after rmTBI. Mol Ther 28(2):503–522. https://doi.org/10.1016/j.ymthe.2019.11.017
Wu Y, Yao J, Feng K (2020) miR-124-5p/NOX2 axis modulates the ROS production and the inflammatory microenvironment to protect against the cerebral I/R injury. Neurochem Res 45(2):404–417. https://doi.org/10.1007/s11064-019-02931-0
Li Z et al (2021) M2 microglial small extracellular vesicles reduce glial scar formation via the miR-124/STAT3 pathway after ischemic stroke in mice. Theranostics 11(3):1232–1248. https://doi.org/10.7150/thno.48761
Woodbury ME et al (2015) miR-155 is essential for inflammation-induced hippocampal neurogenic dysfunction. J Neurosci 35(26):9764–9781. https://doi.org/10.1523/JNEUROSCI.4790-14.2015
Butovsky O et al (2015) Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann Neurol 77(1):75–99. https://doi.org/10.1002/ana.24304
Junker A et al (2009) MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 132(Pt 12):3342–3352. https://doi.org/10.1093/brain/awp300
Cardoso AL et al (2012) miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 135(1):73–88. https://doi.org/10.1111/j.1365-2567.2011.03514.x
Yin H, Song S, Pan X (2017) Knockdown of miR-155 protects microglia against LPS-induced inflammatory injury via targeting RACK1: a novel research for intracranial infection. J Inflamm (Lond) 14:17. https://doi.org/10.1186/s12950-017-0162-7
Guedes JR et al (2014) Early miR-155 upregulation contributes to neuroinflammation in Alzheimer’s disease triple transgenic mouse model. Hum Mol Genet 23(23):6286–6301. https://doi.org/10.1093/hmg/ddu348
Li P et al (2019) MicroRNA-155 promotes heat stress-induced inflammation via targeting liver X receptor alpha in microglia. Front Cell Neurosci 13:12. https://doi.org/10.3389/fncel.2019.00012
Llorens F et al (2017) MicroRNA expression in the locus coeruleus, entorhinal cortex, and hippocampus at early and middle stages of braak neurofibrillary tangle pathology. J Mol Neurosci 63(2):206–215. https://doi.org/10.1007/s12031-017-0971-4
Saba R et al (2012) MicroRNA 146a (miR-146a) is over-expressed during prion disease and modulates the innate immune response and the microglial activation state. PLoS ONE 7(2):e30832. https://doi.org/10.1371/journal.pone.0030832
Butovsky O et al (2012) Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest 122(9):3063–3087. https://doi.org/10.1172/JCI62636
Liang C et al (2021) MicroRNA-146a switches microglial phenotypes to resist the pathological processes and cognitive degradation of Alzheimer’s disease. Theranostics 11(9):4103–4121. https://doi.org/10.7150/thno.53418
Song J, Oh Y, Lee JE (2015) miR-Let7A Modulates Autophagy Induction in LPS-Activated Microglia. Exp Neurobiol 24(2):117–125. https://doi.org/10.5607/en.2015.24.2.117
Li Y et al (2019) Mir223 restrains autophagy and promotes CNS inflammation by targeting ATG16L1. Autophagy 15(3):478–492. https://doi.org/10.1080/15548627.2018.1522467
Zhang L et al (2012) miR-21 represses FasL in microglia and protects against microglia-mediated neuronal cell death following hypoxia/ischemia. Glia 60(12):1888–1895. https://doi.org/10.1002/glia.22404
Yao H et al (2014) MiR-9 promotes microglial activation by targeting MCPIP1. Nat Commun 5:4386. https://doi.org/10.1038/ncomms5386
Brites D (2020) Regulatory function of microRNAs in microglia. Glia 68(8):1631–1642. https://doi.org/10.1002/glia.23846
Guo Y et al (2019) MicroRNAs in microglia: how do MicroRNAs affect activation, inflammation, polarization of microglia and mediate the interaction between microglia and glioma? Front Mol Neurosci 12:125. https://doi.org/10.3389/fnmol.2019.00125
Ye Y et al (2018) A lincRNA-p21/miR-181 family feedback loop regulates microglial activation during systemic LPS- and MPTP- induced neuroinflammation. Cell Death Dis 9(8):803. https://doi.org/10.1038/s41419-018-0821-5
Cai LJ et al (2020) LncRNA MALAT1 facilitates inflammasome activation via epigenetic suppression of Nrf2 in Parkinson’s disease. Mol Brain 13(1):130. https://doi.org/10.1186/s13041-020-00656-8
Mathy NW et al (2021) A novel long intergenic non-coding RNA, Nostrill, regulates iNOS gene transcription and neurotoxicity in microglia. J Neuroinflamm 18(1):16. https://doi.org/10.1186/s12974-020-02051-5
Cao B et al (2018) Long noncoding RNA SNHG1 promotes neuroinflammation in Parkinson’s disease via regulating miR-7/NLRP3 pathway. Neuroscience 388:118–127. https://doi.org/10.1016/j.neuroscience.2018.07.019
Ashwal-Fluss R et al (2014) circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 56(1):55–66. https://doi.org/10.1016/j.molcel.2014.08.019
Rybak-Wolf A et al (2015) Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell 58(5):870–885. https://doi.org/10.1016/j.molcel.2015.03.027
Kulcheski FR, Christoff AP, Margis R (2016) Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J Biotechnol 238:42–51. https://doi.org/10.1016/j.jbiotec.2016.09.011
Curry-Hyde A et al (2020) Cell type-specific circular RNA expression in human glial cells. Genomics 112(6):5265–5274. https://doi.org/10.1016/j.ygeno.2020.09.042
Zhang Y et al (2017) Microarray analysis of circular RNA expression patterns in polarized macrophages. Int J Mol Med 39(2):373–379. https://doi.org/10.3892/ijmm.2017.2852
Ng WL et al (2016) Inducible RasGEF1B circular RNA is a positive regulator of ICAM-1 in the TLR4/LPS pathway. RNA Biol 13(9):861–871. https://doi.org/10.1080/15476286.2016.1207036
Dube U et al (2019) An atlas of cortical circular RNA expression in Alzheimer disease brains demonstrates clinical and pathological associations. Nat Neurosci 22(11):1903–1912. https://doi.org/10.1038/s41593-019-0501-5
Jiang F et al (2022) Circ_0000518 promotes macrophage/microglia M1 polarization via the FUS/CaMKKbeta/AMPK pathway to aggravate multiple sclerosis. Neuroscience 490:131–143. https://doi.org/10.1016/j.neuroscience.2021.12.012
Zhang T et al (2021) The emerging role of exosomes in Alzheimer’s disease. Ageing Res Rev 68:101321. https://doi.org/10.1016/j.arr.2021.101321
Vaz AR et al (2019) Phenotypic effects of wild-type and mutant SOD1 expression in N9 murine microglia at steady state, inflammatory and immunomodulatory conditions. Front Cell Neurosci 13:109. https://doi.org/10.3389/fncel.2019.00109
Herrmann IK, Wood MJA, Fuhrmann G (2021) Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol 16(7):748–759. https://doi.org/10.1038/s41565-021-00931-2
Thompson AG et al (2016) Extracellular vesicles in neurodegenerative disease-pathogenesis to biomarkers. Nat Rev Neurol 12(6):346–357. https://doi.org/10.1038/nrneurol.2016.68
Song Y et al (2019) M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 9(10):2910–2923. https://doi.org/10.7150/thno.30879
Kojima R et al (2018) Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Commun 9(1):1305. https://doi.org/10.1038/s41467-018-03733-8
Duan L et al (2021) Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale 13(3):1387–1397. https://doi.org/10.1039/d0nr07622h
Klemm SL, Shipony Z, Greenleaf WJ (2019) Chromatin accessibility and the regulatory epigenome. Nat Rev Genet 20(4):207–220. https://doi.org/10.1038/s41576-018-0089-8
Jones PL et al (1998) Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet 19(2):187–191. https://doi.org/10.1038/561
Cronk JC et al (2015) Methyl-CpG binding protein 2 regulates microglia and macrophage gene expression in response to inflammatory stimuli. Immunity 42(4):679–691. https://doi.org/10.1016/j.immuni.2015.03.013
Hansen TB et al (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495(7441):384–388. https://doi.org/10.1038/nature11993
Goodall GJ, Wickramasinghe VO (2021) RNA in cancer. Nat Rev Cancer 21(1):22–36. https://doi.org/10.1038/s41568-020-00306-0
Cryan JF et al (2020) The gut microbiome in neurological disorders. Lancet Neurol 19(2):179–194. https://doi.org/10.1016/S1474-4422(19)30356-4
Qian XH et al (2021) Inflammatory pathways in Alzheimer’s disease mediated by gut microbiota. Ageing Res Rev 68:101317. https://doi.org/10.1016/j.arr.2021.101317
Erny D et al (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18(7):965–977. https://doi.org/10.1038/nn.4030
Sampson TR et al (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167(6):1469-1480 e12. https://doi.org/10.1016/j.cell.2016.11.018
Hansen DV, Hanson JE, Sheng M (2018) Microglia in Alzheimer’s disease. J Cell Biol 217(2):459–472. https://doi.org/10.1083/jcb.201709069
Bertram L et al (2008) Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am J Hum Genet 83(5):623–632. https://doi.org/10.1016/j.ajhg.2008.10.008
Yu JT et al (2014) Triggering receptor expressed on myeloid cells 2 variant is rare in late-onset Alzheimer’s disease in Han Chinese individuals. Neurobiol Aging 35(4):937 e1–3. https://doi.org/10.1016/j.neurobiolaging.2013.10.075
Wang P et al (2018) Lack of association between triggering receptor expressed on myeloid cells 2 polymorphism rs75932628 and late-onset Alzheimer’s disease in a Chinese Han population. Psychiatr Genet 28(1):16–18. https://doi.org/10.1097/YPG.0000000000000188
Ma J et al (2014) Association study of TREM2 polymorphism rs75932628 with late-onset Alzheimer’s disease in Chinese Han population. Neurol Res 36(10):894–896. https://doi.org/10.1179/1743132814Y.0000000376
Wang S et al (2020) Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J Exp Med. https://doi.org/10.1084/jem.20200785
Gratuze M, Leyns CEG, Holtzman DM (2018) New insights into the role of TREM2 in Alzheimer’s disease. Mol Neurodegener 13(1):66. https://doi.org/10.1186/s13024-018-0298-9
Griciuc A et al (2013) Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78(4):631–643. https://doi.org/10.1016/j.neuron.2013.04.014
Griciuc A et al (2019) TREM2 acts downstream of CD33 in modulating microglial pathology in Alzheimer’s disease. Neuron 103(5):820-835 e7. https://doi.org/10.1016/j.neuron.2019.06.010
Wissfeld J et al (2021) Reporter cell assay for human CD33 validated by specific antibodies and human iPSC-derived microglia. Sci Rep 11(1):13462. https://doi.org/10.1038/s41598-021-92434-2
Deming Y et al (2019) The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aau2291
Tansey KE, Cameron D, Hill MJ (2018) Genetic risk for Alzheimer’s disease is concentrated in specific macrophage and microglial transcriptional networks. Genome Med 10(1):14. https://doi.org/10.1186/s13073-018-0523-8
McQuade A, Blurton-Jones M (2019) Microglia in Alzheimer’s disease: exploring how genetics and phenotype influence risk. J Mol Biol 431(9):1805–1817. https://doi.org/10.1016/j.jmb.2019.01.045
Wu KM et al (2021) The role of the immune system in Alzheimer’s disease. Ageing Res Rev 70:101409. https://doi.org/10.1016/j.arr.2021.101409
Habes M et al (2021) The Brain Chart of Aging: Machine-learning analytics reveals links between brain aging, white matter disease, amyloid burden, and cognition in the iSTAGING consortium of 10,216 harmonized MR scans. Alzheimers Dement 17(1):89–102. https://doi.org/10.1002/alz.12178
Lu Y et al (2020) Reprogramming to recover youthful epigenetic information and restore vision. Nature 588(7836):124–129. https://doi.org/10.1038/s41586-020-2975-4
Fisher EMC, Bannerman DM (2019) Mouse models of neurodegeneration: know your question, know your mouse. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaq1818
Smith AM, Dragunow M (2014) The human side of microglia. Trends Neurosci 37(3):125–135. https://doi.org/10.1016/j.tins.2013.12.001
Masuda T et al (2019) Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566(7744):388–392. https://doi.org/10.1038/s41586-019-0924-x
Gosselin D et al (2017) An environment-dependent transcriptional network specifies human microglia identity. Science. https://doi.org/10.1126/science.aal3222
Maloney B et al (2007) Important differences between human and mouse APOE gene promoters: limitation of mouse APOE model in studying Alzheimer’s disease. J Neurochem 103(3):1237–1257. https://doi.org/10.1111/j.1471-4159.2007.04831.x
Friedman BA et al (2018) Diverse brain myeloid expression profiles reveal distinct microglial activation states and aspects of Alzheimer’s disease not evident in mouse models. Cell Rep 22(3):832–847. https://doi.org/10.1016/j.celrep.2017.12.066
Takahashi K et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872. https://doi.org/10.1016/j.cell.2007.11.019
Tang Y et al (2017) Direct reprogramming rather than iPSC-based reprogramming maintains aging hallmarks in human motor neurons. Front Mol Neurosci 10:359. https://doi.org/10.3389/fnmol.2017.00359
Chen SW et al (2021) Efficient conversion of human induced pluripotent stem cells into microglia by defined transcription factors. Stem Cell Rep 16(5):1363–1380. https://doi.org/10.1016/j.stemcr.2021.03.010
McQuade A et al (2018) Development and validation of a simplified method to generate human microglia from pluripotent stem cells. Mol Neurodegener 13(1):67. https://doi.org/10.1186/s13024-018-0297-x
Claes C et al (2019) Human stem cell-derived monocytes and microglia-like cells reveal impaired amyloid plaque clearance upon heterozygous or homozygous loss of TREM2. Alzheimers Dement 15(3):453–464. https://doi.org/10.1016/j.jalz.2018.09.006
Mancuso R et al (2019) Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nat Neurosci 22(12):2111–2116. https://doi.org/10.1038/s41593-019-0525-x
Douvaras P et al (2017) Directed differentiation of human pluripotent stem cells to microglia. Stem Cell Rep 8(6):1516–1524. https://doi.org/10.1016/j.stemcr.2017.04.023
Haenseler W et al (2017) A highly efficient human pluripotent stem cell microglia model displays a neuronal-Co-culture-specific expression profile and inflammatory response. Stem Cell Rep 8(6):1727–1742. https://doi.org/10.1016/j.stemcr.2017.05.017
Muffat J et al (2016) Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med 22(11):1358–1367. https://doi.org/10.1038/nm.4189
Pandya H et al (2017) Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat Neurosci 20(5):753–759. https://doi.org/10.1038/nn.4534
Xu R et al (2020) Human iPSC-derived mature microglia retain their identity and functionally integrate in the chimeric mouse brain. Nat Commun 11(1):1577. https://doi.org/10.1038/s41467-020-15411-9
Pocock JM, Piers TM (2018) Modelling microglial function with induced pluripotent stem cells: an update. Nat Rev Neurosci 19(8):445–452. https://doi.org/10.1038/s41583-018-0030-3
Speicher AM et al (2019) Generating microglia from human pluripotent stem cells: novel in vitro models for the study of neurodegeneration. Mol Neurodegener 14(1):46. https://doi.org/10.1186/s13024-019-0347-z
Piers TM et al (2020) A locked immunometabolic switch underlies TREM2 R47H loss of function in human iPSC-derived microglia. FASEB J 34(2):2436–2450. https://doi.org/10.1096/fj.201902447R
Garcia-Reitboeck P et al (2018) Human induced pluripotent stem cell-derived microglia-like cells harboring TREM2 missense mutations show specific deficits in phagocytosis. Cell Rep 24(9):2300–2311. https://doi.org/10.1016/j.celrep.2018.07.094
Brownjohn PW et al (2018) Functional studies of missense TREM2 mutations in human stem cell-derived microglia. Stem Cell Rep 10(4):1294–1307. https://doi.org/10.1016/j.stemcr.2018.03.003
Liu T et al (2020) Multi-omic comparison of Alzheimer’s variants in human ESC-derived microglia reveals convergence at APOE. J Exp Med. https://doi.org/10.1084/jem.20200474
Konttinen H et al (2019) PSEN1DeltaE9, APPswe, and APOE4 confer disparate phenotypes in human iPSC-derived microglia. Stem Cell Rep 13(4):669–683. https://doi.org/10.1016/j.stemcr.2019.08.004
Acknowledgements
We apologize to the authors whose work might have been unintentionally omitted due to space limitations. We would like to thank Dr. Hao Deng from the third Xiangya Hospital of Central South University for providing helpful discussions.
Funding
This study was funded by the National Key R&D Program of China [No. 2022ZD0213700], National Natural Sciences Foundation of China [No. 81801200 to YT; 81901223 to FY], Hunan Provincial Natural Science Foundation of China [No. 2019JJ40476 to YT; 2022JJ40824 to JW], Talents Startup Fund [No. 2209090550 to YT] and Youth Science Foundation [No. 2021Q04 to JW] of Xiangya Hospital, Central South University, Changsha, China.
Author information
Authors and Affiliations
Contributions
YT conceived this study. CL and YT prepared the draft and figures. YT, CL, JR, MZ, HW, FY and JW discussed and revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interest exists.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Ren, J., Zhang, M. et al. The heterogeneity of microglial activation and its epigenetic and non-coding RNA regulations in the immunopathogenesis of neurodegenerative diseases. Cell. Mol. Life Sci. 79, 511 (2022). https://doi.org/10.1007/s00018-022-04536-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04536-3