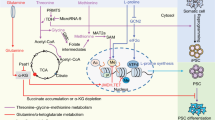
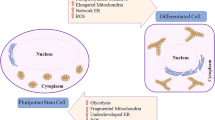
Abstract
All living organisms need energy to carry out their essential functions. The importance of energy metabolism is increasingly recognized in human pluripotent stem cells. Energy production is not only essential for cell survival and proliferation, but also critical for pluripotency and cell fate determination. Thus, energy metabolism is an important target in cellular regulation and stem cell applications. In this review, we will discuss key factors that influence energy metabolism and their association with stem cell functions.


Similar content being viewed by others
Availability of data and material
Not applicable.
References
Accili D, Arden KC (2004) FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117(4):421–426
Aft RL, Zhang FW, Gius D (2002) Evaluation of 2-deoxy-d-glucose as a chemotherapeutic agent: mechanism of cell death. Br J Cancer 87(7):805–812
Alvarez-Tejado M, Naranjo-Suarez S, Jimenez C, Carrera AC, Landazuri MO, del Peso L (2001) Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J Biol Chem 276(25):22368–22374
Arcidiacono B, Chiefari E, Messineo S, Bilotta FL, Pastore I, Corigliano DM, Foti DP, Brunetti A (2018) HMGA1 is a novel transcriptional regulator of the FoxO1 gene. Endocrine 60(1):56–64
Arthur SA, Blaydes JP, Houghton FD (2019) Glycolysis regulates human embryonic stem cell self-renewal under hypoxia through HIF-2alpha and the glycolytic sensors CTBPs. Stem Cell Rep 12(4):728–742
Birket MJ, Orr AL, Gerencser AA, Madden DT, Vitelli C, Swistowski A, Brand MD, Zeng X (2011) A reduction in ATP demand and mitochondrial activity with neural differentiation of human embryonic stem cells. J Cell Sci 124(Pt 3):348–358
Boedtkjer E, Praetorius J, Matchkov VV, Stankevicius E, Mogensen S, Fuchtbauer AC, Simonsen U, Fuchtbauer EM, Aalkjaer C (2011) Disruption of Na+, HCO(3)(−) cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca(2)(+) sensitivity, and hypertension development in mice. Circulation 124(17):1819–1829
Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED (2007) A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11(1):37–51
Buchholz DE, Pennington BO, Croze RH, Hinman CR, Coffey PJ, Clegg DO (2013) Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl Med 2(5):384–393
Carbognin E, Betto RM, Soriano ME, Smith AG, Martello G (2016) Stat3 promotes mitochondrial transcription and oxidative respiration during maintenance and induction of naive pluripotency. EMBO J 35(6):618–634
Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB (2015) Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518(7539):413–416
Casey JR, Grinstein S, Orlowski J (2010) Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11(1):50–61
Chakrabarty RP, Chandel NS (2021) Mitochondria as signaling organelles control mammalian stem cell fate. Cell Stem Cell 28(3):394–408
Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27(3):275–280
Chen GK, Gulbranson DR, Hou ZG, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JMC, Thomson JA (2011) Chemically defined conditions for human iPSC derivation and culture. Nat Methods 8(5):424-U476
Chen JL, Lucas JE, Schroeder T, Mori S, Wu J, Nevins J, Dewhirst M, West M, Chi JT (2008) The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genet 4(12):e1000293
Chen W, Gueron M (1992) The inhibition of bovine heart hexokinase by 2-deoxy-d-glucose-6-phosphate: characterization by 31P NMR and metabolic implications. Biochimie 74(9–10):867–873
Chiefari E, Foti DP, Sgarra R, Pegoraro S, Arcidiacono B, Brunetti FS, Greco M, Manfioletti G, Brunetti A (2018) Transcriptional regulation of glucose metabolism: the emerging role of the HMGA1 chromatin factor. Front Endocrinol (Lausanne) 9:357
Chiefari E, Nevolo MT, Arcidiacono B, Maurizio E, Nocera A, Iiritano S, Sgarra R, Possidente K, Palmieri C, Paonessa F, Brunetti G, Manfioletti G, Foti D, Brunetti A (2012) HMGA1 is a novel downstream nuclear target of the insulin receptor signaling pathway. Sci Rep 2:251
Choudhry H, Harris AL (2018) Advances in hypoxia-inducible factor biology. Cell Metab 27(2):281–298
Christensen DR, Calder PC, Houghton FD (2015) GLUT3 and PKM2 regulate OCT4 expression and support the hypoxic culture of human embryonic stem cells. Sci Rep 5:17500
Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, Terzic A (2007) Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med 4(Suppl 1):S60-67
Cornacchia D, Zhang C, Zimmer B, Chung SY, Fan Y, Soliman MA, Tchieu J, Chambers SM, Shah H, Paull D, Konrad C, Vincendeau M, Noggle SA, Manfredi G, Finley LWS, Cross JR, Betel D, Studer L (2019) Lipid deprivation induces a stable, naive-to-primed intermediate state of pluripotency in human PSCs. Cell Stem Cell 25(1):120-136 e110
Dahan P, Lu V, Nguyen RMT, Kennedy SAL, Teitell MA (2019) Metabolism in pluripotency: both driver and passenger? J Biol Chem 294(14):5420–5429
Dai Z, Ramesh V, Locasale JW (2020) The evolving metabolic landscape of chromatin biology and epigenetics. Nat Rev Genet 21(12):737–753
Eagle H (1971) Buffer combinations for mammalian cell culture. Science 174(4008):500–503
Ebeling P, Koistinen HA, Koivisto VA (1998) Insulin-independent glucose transport regulates insulin sensitivity. FEBS Lett 436(3):301–303
Etchegaray JP, Mostoslavsky R (2016) Interplay between metabolism and epigenetics: a nuclear adaptation to environmental changes. Mol Cell 62(5):695–711
Ezashi T, Das P, Roberts RM (2005) Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA 102(13):4783–4788
Fang Y, Liu Z, Chen Z, Xu X, Xiao M, Yu Y, Zhang Y, Zhang X, Du Y, Jiang C, Zhao Y, Wang Y, Fan B, Terheyden-Keighley D, Liu Y, Shi L, Hui Y, Zhang X, Zhang B, Feng H, Ma L, Zhang Q, Jin G, Yang Y, Xiang B, Liu L, Zhang X (2017) Smad5 acts as an intracellular pH messenger and maintains bioenergetic homeostasis. Cell Res 27(9):1083–1099
Folmes CD, Dzeja PP, Nelson TJ, Terzic A (2012) Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell 11(5):596–606
Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A (2011) Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab 14(2):264–271
Forristal CE, Christensen DR, Chinnery FE, Petruzzelli R, Parry KL, Sanchez-Elsner T, Houghton FD (2013) Environmental oxygen tension regulates the energy metabolism and self-renewal of human embryonic stem cells. PLoS ONE 8(5):e62507
Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD (2010) Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 139(1):85–97
Foti D, Chiefari E, Fedele M, Iuliano R, Brunetti L, Paonessa F, Manfioletti G, Barbetti F, Brunetti A, Croce CM, Fusco A, Brunetti A (2005) Lack of the architectural factor HMGA1 causes insulin resistance and diabetes in humans and mice. Nat Med 11(7):765–773
Fukamachi T, Ikeda S, Wang X, Saito H, Tagawa M, Kobayashi H (2013) Gene expressions for signal transduction under acidic conditions. Genes (Basel) 4(1):65–85
Godoy-Parejo C, Deng C, Zhang Y, Liu W, Chen G (2020) Roles of vitamins in stem cells. Cell Mol Life Sci 77(9):1771–1791
Graves CN, Biggers JD (1970) Carbon dioxide fixation by mouse embryos prior to implantation. Science 167(3924):1506–1508
Gross DN, van den Heuvel AP, Birnbaum MJ (2008) The role of FoxO in the regulation of metabolism. Oncogene 27(16):2320–2336
Gu W, Gaeta X, Sahakyan A, Chan AB, Hong CS, Kim R, Braas D, Plath K, Lowry WE, Christofk HR (2016) Glycolytic metabolism plays a functional role in regulating human pluripotent stem cell state. Cell Stem Cell 19(4):476–490
Guo G, von Meyenn F, Rostovskaya M, Clarke J, Dietmann S, Baker D, Sahakyan A, Myers S, Bertone P, Reik W, Plath K, Smith A (2017) Epigenetic resetting of human pluripotency. Development 144(15):2748–2763
Halestrap AP (1975) The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem J 148(1):85–96
Halestrap AP, Meredith D (2004) The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch 447(5):619–628
Halperin ML, Connors HP, Relman AS, Karnovsky ML (1969) Factors that control the effect of pH on glycolysis in leukocytes. J Biol Chem 244(2):384–390
Johannsen DL, Ravussin E (2009) The role of mitochondria in health and disease. Curr Opin Pharmacol 9(6):780–786
Jung J, Zeng H, Horng T (2019) Metabolism as a guiding force for immunity. Nat Cell Biol 21(1):85–93
Kaelin WG Jr, Ratcliffe PJ (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30(4):393–402
Kaminskas E (1978) The pH-dependence of sugar-transport and glycolysis in cultured Ehrlich ascites-tumour cells. Biochem J 174(2):453–459
Kargaran PK, Mosqueira D, Kozicz T (2020) Mitochondrial medicine: genetic underpinnings and disease modeling using induced pluripotent stem cell technology. Front Cardiovasc Med 7:604581
Kierans SJ, Taylor CT (2021) Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J Physiol 599(1):23–37
Kim JW, Tchernyshyov I, Semenza GL, Dang CV (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3(3):177–185
Kulkarni V, Khadilkar RJ, Magadi SS, Inamdar MS (2011) Asrij maintains the stem cell niche and controls differentiation during Drosophila lymph gland hematopoiesis. PLoS ONE 6(11):e27667
Kuwata F, Suzuki N, Otsuka K, Taguchi M, Sasai Y, Wakino H, Ito M, Ebihara S, Suzuki K (1991) Enzymatic regulation of glycolysis and gluconeogenesis in rabbit periodontal ligament under various physiological pH conditions. J Nihon Univ Sch Dent 33(2):81–90
Laszlo J, Humphreys SR, Goldin A (1960) Effects of glucose analogues (2-deoxy-d-glucose, 2-deoxy-d-galactose) on experimental tumors. J Natl Cancer Inst 24:267–281
Leese HJ, Barton AM (1984) Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J Reprod Fertil 72(1):9–13
Levitzki A, Koshland DE Jr (1971) Cytidine triphosphate synthetase. Covalent intermediates and mechanisms of action. Biochemistry 10(18):3365–3371
Liu W, Deng C, Godoy-Parejo C, Zhang Y, Chen G (2019) Developments in cell culture systems for human pluripotent stem cells. World J Stem Cells 11(11):968–981
Liu W, Ren Z, Lu K, Song C, Cheung ECW, Zhou Z, Chen G (2018) The suppression of medium acidosis improves the maintenance and differentiation of human pluripotent stem cells at high density in defined cell culture medium. Int J Biol Sci 14(5):485–496
Ludikhuize MC, Rodriguez Colman MJ (2021) Metabolic regulation of stem cells and differentiation: a Forkhead box o transcription factor perspective. Antioxid Redox Signal 34(13):1004–1024
Luna LA, Lesecq Z, White KA, Hoang A, Scott DA, Zagnitko O, Bobkov AA, Barber DL, Schiffer JM, Isom DG, Sohl CD (2020) An acidic residue buried in the dimer interface of isocitrate dehydrogenase 1 (IDH1) helps regulate catalysis and pH sensitivity. Biochem J 477(16):2999–3018
Lunt SY, Vander Heiden MG (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27:441–464
Mackenzie CG, Mackenzie JB, Beck P (1961) The effect of pH on growth, protein synthesis, and lipid-rich particles of cultured mammalian cells. J Biophys Biochem Cytol 9:141–156
Mandal S, Lindgren AG, Srivastava AS, Clark AT, Banerjee U (2011) Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells 29(3):486–495
Marsboom G, Zhang GF, Pohl-Avila N, Zhang Y, Yuan Y, Kang H, Hao B, Brunengraber H, Malik AB, Rehman J (2016) Glutamine metabolism regulates the pluripotency transcription factor OCT4. Cell Rep 16(2):323–332
Mas-Bargues C, Sanz-Ros J, Roman-Dominguez A, Ingles M, Gimeno-Mallench L, El Alami M, Vina-Almunia J, Gambini J, Vina J, Borras C (2019) Relevance of oxygen concentration in stem cell culture for regenerative medicine. Int J Mol Sci 20(5):1195
Mathieu J, Ruohola-Baker H (2017) Metabolic remodeling during the loss and acquisition of pluripotency. Development 144(4):541–551
McCommis KS, Finck BN (2015) Mitochondrial pyruvate transport: a historical perspective and future research directions. Biochem J 466(3):443–454
Michl J, Park KC, Swietach P (2019) Evidence-based guidelines for controlling pH in mammalian live-cell culture systems. Commun Biol 2:144
Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D, Nemirovski A, Shen-Orr S, Laevsky I, Amit M, Bomze D, Elena-Herrmann B, Scherf T, Nissim-Rafinia M, Kempa S, Itskovitz-Eldor J, Meshorer E, Aberdam D, Nahmias Y (2015) Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab 21(3):392–402
Mylonis I, Simos G, Paraskeva E (2019) Hypoxia-inducible factors and the regulation of lipid metabolism. Cells 8(3):214
Oburoglu L, Tardito S, Fritz V, de Barros SC, Merida P, Craveiro M, Mamede J, Cretenet G, Mongellaz C, An X, Klysz D, Touhami J, Boyer-Clavel M, Battini JL, Dardalhon V, Zimmermann VS, Mohandas N, Gottlieb E, Sitbon M, Kinet S, Taylor N (2014) Glucose and glutamine metabolism regulate human hematopoietic stem cell lineage specification. Cell Stem Cell 15(2):169–184
Ochocki JD, Simon MC (2013) Nutrient-sensing pathways and metabolic regulation in stem cells. J Cell Biol 203(1):23–33
Oginuma M, Harima Y, Tarazona OA, Diaz-Cuadros M, Michaut A, Ishitani T, Xiong F, Pourquie O (2020) Intracellular pH controls WNT downstream of glycolysis in amniote embryos. Nature 584(7819):98–101
Panopoulos AD, Yanes O, Ruiz S, Kida YS, Diep D, Tautenhahn R, Herrerias A, Batchelder EM, Plongthongkum N, Lutz M, Berggren WT, Zhang K, Evans RM, Siuzdak G, Izpisua Belmonte JC (2012) The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res 22(1):168–177
Parisi S, Piscitelli S, Passaro F, Russo T (2020) HMGA proteins in stemness and differentiation of embryonic and adult stem cells. Int J Mol Sci 21(1):362
Pereira SL, Graos M, Rodrigues AS, Anjo SI, Carvalho RA, Oliveira PJ, Arenas E, Ramalho-Santos J (2013) Inhibition of mitochondrial complex III blocks neuronal differentiation and maintains embryonic stem cell pluripotency. PLoS ONE 8(12):e82095
Quach CH, Jung KH, Lee JH, Park JW, Moon SH, Cho YS, Choe YS, Lee KH (2016) Mild alkalization acutely triggers the warburg effect by enhancing hexokinase activity via voltage-dependent anion channel binding. PLoS ONE 11(8):e0159529
Ratcliffe PJ (2013) Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J Physiol 591(8):2027–2042
Relman AS (1972) Metabolic consequences of acid-base disorders. Kidney Int 1(5):347–359
Ren Z, Zhong H, Song C, Deng C, Hsieh HT, Liu W, Chen G (2020) Insulin promotes mitochondrial respiration and survival through PI3K/AKT/GSK3 pathway in human embryonic stem cells. Stem Cell Rep 15(6):1362–1376
Riddle SR, Ahmad A, Ahmad S, Deeb SS, Malkki M, Schneider BK, Allen CB, White CW (2000) Hypoxia induces hexokinase II gene expression in human lung cell line A549. Am J Physiol Lung Cell Mol Physiol 278(2):L407-416
Ryall JG, Cliff T, Dalton S, Sartorelli V (2015) Metabolic reprogramming of stem cell epigenetics. Cell Stem Cell 17(6):651–662
Schieke SM, Ma M, Cao L, McCoy JP Jr, Liu C, Hensel NF, Barrett AJ, Boehm M, Finkel T (2008) Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J Biol Chem 283(42):28506–28512
Seifter JL (2019) Body fluid compartments, cell membrane ion transport, electrolyte concentrations, and acid-base balance. Semin Nephrol 39(4):368–379
Semenza GL (2011) Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta 1813(7):1263–1268
Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A (1996) Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271(51):32529–32537
Serra M, Brito C, Sousa MF, Jensen J, Tostoes R, Clemente J, Strehl R, Hyllner J, Carrondo MJ, Alves PM (2010) Improving expansion of pluripotent human embryonic stem cells in perfused bioreactors through oxygen control. J Biotechnol 148(4):208–215
Shah SN, Kerr C, Cope L, Zambidis E, Liu C, Hillion J, Belton A, Huso DL, Resar LM (2012) HMGA1 reprograms somatic cells into pluripotent stem cells by inducing stem cell transcriptional networks. PLoS ONE 7(11):e48533
Shen YC, Ou DL, Hsu C, Lin KL, Chang CY, Lin CY, Liu SH, Cheng AL (2013) Activating oxidative phosphorylation by a pyruvate dehydrogenase kinase inhibitor overcomes sorafenib resistance of hepatocellular carcinoma. Br J Cancer 108(1):72–81
Shetty DK, Kalamkar KP, Inamdar MS (2018) OCIAD1 controls electron transport chain complex I activity to regulate energy metabolism in human pluripotent stem cells. Stem Cell Rep 11(1):128–141
Shyh-Chang N, Daley GQ, Cantley LC (2013) Stem cell metabolism in tissue development and aging. Development 140(12):2535–2547
Shyh-Chang N, Ng HH (2017) The metabolic programming of stem cells. Genes Dev 31(4):336–346
Sinha A, Khadilkar RJ, Vinay KS, Roychowdhury Sinha A, Inamdar MS (2013) Conserved regulation of the Jak/STAT pathway by the endosomal protein asrij maintains stem cell potency. Cell Rep 4(4):649–658
Song C, Xu F, Ren Z, Zhang Y, Meng Y, Yang Y, Lingadahalli S, Cheung E, Li G, Liu W, Wan J, Zhao Y, Chen G (2019) Elevated exogenous pyruvate potentiates mesodermal differentiation through metabolic modulation and AMPK/mTOR pathway in human embryonic stem cells. Stem Cell Rep 13(2):338–351
Sperber H, Mathieu J, Wang Y, Ferreccio A, Hesson J, Xu Z, Fischer KA, Devi A, Detraux D, Gu H, Battle SL, Showalter M, Valensisi C, Bielas JH, Ericson NG, Margaretha L, Robitaille AM, Margineantu D, Fiehn O, Hockenbery D, Blau CA, Raftery D, Margolin AA, Hawkins RD, Moon RT, Ware CB, Ruohola-Baker H (2015) The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nat Cell Biol 17(12):1523–1535
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Tohyama S, Fujita J, Hishiki T, Matsuura T, Hattori F, Ohno R, Kanazawa H, Seki T, Nakajima K, Kishino Y, Okada M, Hirano A, Kuroda T, Yasuda S, Sato Y, Yuasa S, Sano M, Suematsu M, Fukuda K (2016) Glutamine oxidation is indispensable for survival of human pluripotent stem cells. Cell Metab 23(4):663–674
Trivedi B, Danforth WH (1966) Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem 241(17):4110–4112
Tsogtbaatar E, Landin C, Minter-Dykhouse K, Folmes CDL (2020) Energy metabolism regulates stem cell pluripotency. Front Cell Dev Biol 8:87
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033
Vander Heiden MG, DeBerardinis RJ (2017) Understanding the intersections between metabolism and cancer biology. Cell 168(4):657–669
Varum S, Momcilovic O, Castro C, Ben-Yehudah A, Ramalho-Santos J, Navara CS (2009) Enhancement of human embryonic stem cell pluripotency through inhibition of the mitochondrial respiratory chain. Stem Cell Res 3(2–3):142–156
Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley CAT, Ramalho-Santos J, Van Houten B, Schatten G (2011) Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE 6(6):e20914
Weinberger L, Ayyash M, Novershtern N, Hanna JH (2016) Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat Rev Mol Cell Biol 17(3):155–169
Whitehouse S, Cooper RH, Randle PJ (1974) Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem J 141(3):761–774
Wondisford AR, Xiong L, Chang E, Meng S, Meyers DJ, Li M, Cole PA, He L (2014) Control of Foxo1 gene expression by co-activator P300. J Biol Chem 289(7):4326–4333
Yanes O, Clark J, Wong DM, Patti GJ, Sanchez-Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G (2010) Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol 6(6):411–417
Yang Y, Ren Z, Xu F, Meng Y, Zhang Y, Ai N, Long Y, Fok HI, Deng C, Zhao X, Huang L, Zhao Q, Wang J, Liu W, Ge W, Chen G (2019) Endogenous IGF signaling directs heterogeneous mesoderm differentiation in human embryonic stem cells. Cell Rep 29(11):3374-3384 e3375
Yoo HC, Yu YC, Sung Y, Han JM (2020) Glutamine reliance in cell metabolism. Exp Mol Med 52(9):1496–1516
Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S (2009) Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 5(3):237–241
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318(5858):1917–1920
Zhang C, Skamagki M, Liu Z, Ananthanarayanan A, Zhao R, Li H, Kim K (2017) Biological significance of the suppression of oxidative phosphorylation in induced pluripotent stem cells. Cell Rep 21(8):2058–2065
Zhang H, Badur MG, Divakaruni AS, Parker SJ, Jager C, Hiller K, Murphy AN, Metallo CM (2016) Distinct metabolic states can support self-renewal and lipogenesis in human pluripotent stem cells under different culture conditions. Cell Rep 16(6):1536–1547
Zhang J, Nuebel E, Daley GQ, Koehler CM, Teitell MA (2012) Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell 11(5):589–595
Zhang J, Zhao J, Dahan P, Lu V, Zhang C, Li H, Teitell MA (2018) Metabolism in pluripotent stem cells and early mammalian development. Cell Metab 27(2):332–338
Zhang X, Yalcin S, Lee DF, Yeh TY, Lee SM, Su J, Mungamuri SK, Rimmele P, Kennedy M, Sellers R, Landthaler M, Tuschl T, Chi NW, Lemischka I, Keller G, Ghaffari S (2011) FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat Cell Biol 13(9):1092–1099
Zhang Y, Cui P, Li Y, Feng G, Tong M, Guo L, Li T, Liu L, Li W, Zhou Q (2018) Mitochondrially produced ATP affects stem cell pluripotency via Actl6a-mediated histone acetylation. FASEB J 32(4):1891–1902
Zhong H, Ren Z, Wang X, Miao K, Ni W, Meng Y, Lu L, Wang C, Liu W, Deng CX, Xu RH, Chen G (2020) Stagewise keratinocyte differentiation from human embryonic stem cells by defined signal transduction modulators. Int J Biol Sci 16(8):1450–1462
Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, Cavanaugh C, Blau CA, Horwitz MS, Hockenbery D, Ware C, Ruohola-Baker H (2012) HIF1alpha induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J 31(9):2103–2116
Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S (2010) Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 7(6):651–655
Acknowledgements
We thank the council members of Macau Society for Stem Cell Research (MSSCR) for the constructive discussions.
Funding
This work was supported by the University of Macau (File No. MYRG2018-00135-FHS and MYRG2019-00147-FHS), and also by the Science and Technology Development Fund, Macau SAR (File No. 0059/2019/A1, 0123/2019/A3 and 0011/2019/AKP).
Author information
Authors and Affiliations
Contributions
Both authors conceptualized and drafted the article. WL designed the figures. Both authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors consent to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, W., Chen, G. Regulation of energy metabolism in human pluripotent stem cells. Cell. Mol. Life Sci. 78, 8097–8108 (2021). https://doi.org/10.1007/s00018-021-04016-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-04016-0