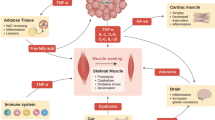
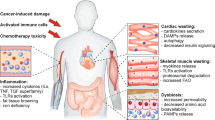
Abstract
Cancer cachexia afflicts many advanced cancer patients with many progressing to death. While there have been many advancements in understanding the molecular mechanisms that contribute to the development of cancer cachexia, substantial gaps still exist. Chemotherapy drugs often target ribosome biogenesis to slow or blunt tumor cell growth and proliferation. Some of the most frequent side-effects of chemotherapy are loss of skeletal muscle mass, muscular strength and an increase in fatigue. Given that ribosome biogenesis has emerged as a main mechanism regulating muscle hypertrophy, and more recently, also implicated in muscle atrophy, we propose that some chemotherapy drugs can cause further muscle wasting via its effect on skeletal muscle cells. Many chemotherapy drugs, including the most prescribed drugs such as doxorubicin and cisplatin, affect ribosomal DNA transcription, or other pathways related to ribosome biogenesis. Furthermore, middle-aged and older individuals are the most affected population with cancer, and advanced cancer patients often show reduced levels of physical inactivity. Thus, aging and inactivity can themselves affect muscle ribosome biogenesis, which can further worsen the effect of chemotherapy on skeletal muscle ribosome biogenesis and, ultimately, muscle mass and function. We propose that chemotherapy can accelerate the onset or worsen cancer cachexia via its inhibitory effects on skeletal muscle ribosome biogenesis. We end our review by providing recommendations that could be used to ameliorate the negative effects of chemotherapy on skeletal muscle ribosome biogenesis.


Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108
Plaza JA, Perez-Montiel D, Mayerson J, Morrison C, Suster S (2008) Metastases to soft tissue: a review of 118 cases over a 30-year period. Cancer 112(1):193–203
Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT et al (2011) Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med 62:265–279
Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G et al (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.” Clin Nutr 29(2):154–159
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12(5):489–495
Blauwhoff-Buskermolen S, de van der Schueren MA, Verheul HM, Langius JA (2014) “Pre-cachexia”: a non-existing phenomenon in cancer? Ann Oncol 25(8):1668–1669
Blum D, Stene GB, Solheim TS, Fayers P, Hjermstad MJ, Baracos VE et al (2014) Validation of the consensus-definition for cancer cachexia and evaluation of a classification model—a study based on data from an international multicentre project (EPCRC-CSA). Ann Oncol 25(8):1635–1642
Pin F, Barreto R, Couch ME, Bonetto A, O’Connell TM (2019) Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle 10(1):140–154
Damrauer JS, Stadler ME, Acharyya S, Baldwin AS, Couch ME, Guttridge DC (2018) Chemotherapy-induced muscle wasting: association with NF-κB and cancer cachexia. Eur J Transl Myol 28(2):7590
Naito T, Okayama T, Aoyama T, Ohashi T, Masuda Y, Kimura M et al (2017) Skeletal muscle depletion during chemotherapy has a large impact on physical function in elderly Japanese patients with advanced non-small-cell lung cancer. BMC Cancer 17(1):571
Daly LE, Ni Bhuachalla EB, Power DG, Cushen SJ, James K, Ryan AM (2018) Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle 9(2):315–325
Thomas G (2000) An encore for ribosome biogenesis in the control of cell proliferation. Nat Cell Biol 2(5):E71–E72
Derenzini M, Montanaro L, Trere D (2017) Ribosome biogenesis and cancer. Acta Histochem 119(3):190–197
Goldstein M, Kastan MB (2015) The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu Rev Med 66:129–143
Bouwman P, Jonkers J (2012) The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer 12(9):587–598
Figueiredo VC, McCarthy JJ (2019) Regulation of ribosome biogenesis in skeletal muscle hypertrophy. Physiology (Bethesda) 34(1):30–42
Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M (2013) Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280(17):4294–4314
von Haehling S, Anker SD (2010) Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle 1(1):1–5
Ruiz JR, Sui X, Lobelo F, Morrow JR, Jackson AW, Sjöström M et al (2008) Association between muscular strength and mortality in men: prospective cohort study. BMJ (Clin. Res Ed) 337:439
Ruiz JR, Sui X, Lobelo F, Lee D-C, Morrow JR, Jackson AW et al (2009) Muscular strength and adiposity as predictors of adulthood cancer mortality in men. Cancer Epidemiol Biomarkers Prev 18(5):1468–1476
Gale CR, Martyn CN, Cooper C, Sayer AA (2007) Grip strength, body composition, and mortality. Int J Epidemiol 36(1):228–235
Villaseñor A, Ballard-Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A et al (2012) Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Survivorship Res Pract 6(4):398–406
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31(12):1539–1547
Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MAE, den Braver NR, Berkhof J, Langius JAE et al (2016) Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 34(12):1339–1344
Metter EJ, Talbot LA, Schrager M, Conwit R (2002) Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 57(10):B359–B365
Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB et al (2006) Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 61(1):72–77
Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K et al (2000) Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci 55(3):M168–M173
Prado CMM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15(8):2920–2926
da Rocha IMG, Marcadenti A, de Medeiros GOC, Bezerra RA, Rego JFM, Gonzalez MC et al (2019) Is cachexia associated with chemotherapy toxicities in gastrointestinal cancer patients? A prospective study. J Cachexia Sarcopenia Muscle 10(2):445–454
Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q et al (2010) Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142(4):531–543
Wannamethee SG, Shaper AG, Lennon L, Whincup PH (2007) Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr 86(5):1339–1346
Mueller TC, Bachmann J, Prokopchuk O, Friess H, Martignoni ME (2016) Molecular pathways leading to loss of skeletal muscle mass in cancer cachexia—can findings from animal models be translated to humans? BMC Cancer 8(16):75
Tisdale MJ (2002) Cachexia in cancer patients. Nat Rev Cancer 2(11):862–871
Smith KL, Tisdale MJ (1993) Increased protein degradation and decreased protein synthesis in skeletal muscle during cancer cachexia. Br J Cancer 67(4):680–685
Samuels SE, Knowles AL, Tilignac T, Debiton E, Madelmont JC, Attaix D (2001) Higher skeletal muscle protein synthesis and lower breakdown after chemotherapy in cachectic mice. Am J Physiol Regul Integr Comp Physiol 281(1):R133–R139
Lundholm K, Bylund AC, Holm J, Scherstén T (1976) Skeletal muscle metabolism in patients with malignant tumor. Eur J Cancer 12(6):465–473
White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S et al (2011) The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS ONE 6(9):e24650
Emery PW, Edwards RH, Rennie MJ, Souhami RL, Halliday D (1984) Protein synthesis in muscle measured in vivo in cachectic patients with cancer. Br Med J (Clin Res Ed) 289(6445):584–586
Lautaoja JH, Lalowski M, Nissinen TA, Hentila J, Shi Y, Ritvos O et al (2019) Muscle and serum metabolomes are dysregulated in colon-26 tumor-bearing mice despite amelioration of cachexia with activin receptor type 2B ligand blockade. Am J Physiol Endocrinol Metab 316(5):E852–E865
Kim HG, Huot JR, Pin F, Guo B, Bonetto A, Nader GA (2021) Reduced rDNA transcription diminishes skeletal muscle ribosomal capacity and protein synthesis in cancer cachexia. FASEB J 35(2):e21335
Preston-Martin S, Pike MC, Ross RK, Jones PA, Henderson BE (1990) Increased cell division as a cause of human cancer. Can Res 50(23):7415–7421
Giancotti FG (2014) Deregulation of cell signaling in cancer. FEBS Lett 588(16):2558–2570
Evan GI, Vousden KH (2001) Proliferation, cell cycle and apoptosis in cancer. Nature 411(6835):342–348
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7(1):11–20
van Riggelen J, Yetil A, Felsher DW (2010) MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer 10(4):301–309
Guertin DA, Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer Cell 12(1):9–22
Bader AG, Vogt PK (2004) An essential role for protein synthesis in oncogenic cellular transformation. Oncogene 23(18):3145–3150
Altomare DA, Testa JR (2005) Perturbations of the AKT signaling pathway in human cancer. Oncogene 24(50):7455–7464
Janku F, Yap TA, Meric-Bernstam F (2018) Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol 15(5):273–291
Henras AK, Plisson-Chastang C, O’Donohue MF, Chakraborty A, Gleizes PE (2015) An overview of pre-ribosomal RNA processing in eukaryotes. Wiley Interdiscipl Rev RNA 6(2):225–242
Drygin D, Rice WG, Grummt I (2010) The RNA polymerase I transcription machinery: an emerging target for the treatment of cancer. Annu Rev Pharmacol Toxicol 50(1):131–156
Mayer C, Grummt I (2006) Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 25(48):6384–6391
van Sluis M, McStay B (2014) Ribosome biogenesis: achilles heel of cancer? Genes Cancer 5(5–6):152–153
Ruggero D, Pandolfi PP (2003) Does the ribosome translate cancer? Nat Rev Cancer 3(3):179–192
Rosenwald IB (2004) The role of translation in neoplastic transformation from a pathologist’s point of view. Oncogene 23(18):3230–3247
Montanaro L, Treré D, Derenzini M (2008) Nucleolus, ribosomes, and cancer. Am J Pathol 173(2):301–310
Burger K, Mühl B, Harasim T, Rohrmoser M, Malamoussi A, Orban M et al (2010) Chemotherapeutic drugs inhibit ribosome biogenesis at various levels. J Biol Chem 285(16):12416–12425
Freed EF, Bleichert F, Dutca LM, Baserga SJ (2010) When ribosomes go bad: diseases of ribosome biogenesis. Mol BioSyst 6(3):481–493
Hannan RD, Drygin D, Pearson RB (2013) Targeting RNA polymerase I transcription and the nucleolus for cancer therapy. Expert Opin Ther Targets 17(8):873–878
Hein N, Hannan KM, George AJ, Sanij E, Hannan RD (2013) The nucleolus: an emerging target for cancer therapy. Trends Mol Med 19(11):643–654
Poortinga G, Quinn LM, Hannan RD (2015) Targeting RNA polymerase I to treat MYC-driven cancer. Oncogene 34(4):403–412
Quin JE, Devlin JR, Cameron D, Hannan KM, Pearson RB, Hannan RD (2014) Targeting the nucleolus for cancer intervention. Biochem Biophys Acta 1842(6):802–816
Tsai RYL, Pederson T (2014) Connecting the nucleolus to the cell cycle and human disease. FASEB J 28(8):3290–3296
Chan JC, Hannan KM, Riddell K, Ng PY, Peck A, Lee RS et al (2011) AKT promotes rRNA synthesis and cooperates with c-MYC to stimulate ribosome biogenesis in cancer. Sci signal 4(188):ra56-ra
Iadevaia V, Liu R, Proud CG (2014) mTORC1 signaling controls multiple steps in ribosome biogenesis. Semin Cell Dev Biol 36:113–120
Guo QM, Malek RL, Kim S, Chiao C, He M, Ruffy M et al (2000) Identification of c-myc responsive genes using rat cDNA microarray. Can Res 60(21):5922–5928
Schlosser I, Hölzel M, Mürnseer M, Burtscher H, Weidle UH, Eick D (2003) A role for c-Myc in the regulation of ribosomal RNA processing. Nucleic Acids Res 31(21):6148–6156
Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K et al (2010) MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther 9(7):1956–1967
Ahn DH, Li J, Wei L, Doyle A, Marshall JL, Schaaf LJ et al (2015) Results of an abbreviated phase-II study with the Akt Inhibitor MK-2206 in patients with advanced biliary cancer. Sci Rep 5:12122
Meng LH, Zheng XF (2015) Toward rapamycin analog (rapalog)-based precision cancer therapy. Acta Pharmacol Sin 36(10):1163–1169
Xie J, Wang X, Proud CG. mTOR inhibitors in cancer therapy. F1000Res. 2016;5
Drygin D, Siddiqui-Jain A, O’Brien S, Schwaebe M, Lin A, Bliesath J et al (2009) Anticancer activity of CX-3543: a direct inhibitor of rRNA biogenesis. Can Res 69(19):7653–7661
O’Brien S, Drygin D, Harrison SJ, Khot A, Cullinane C, Geoff M et al (2013) Inhibition of RNA polymerase I transcription by CX-5461 as a therapeutic strategy for the cancer-specific activation of p53 in highly refractory haematological malignancies. Blood 122(21):3941
Drygin D, Lin A, Bliesath J, Ho CB, O’Brien SE, Proffitt C et al (2011) Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res 71(4):1418–1430
Peltonen K, Colis L, Liu H, Trivedi R, Moubarek MS, Moore HM et al (2014) A targeting modality for destruction of RNA polymerase I that possesses anticancer activity. Cancer Cell 25(1):77–90
Ferreira R, Schneekloth JS Jr, Panov KI, Hannan KM, Hannan RD (2020) Targeting the RNA polymerase I transcription for cancer therapy comes of age. Cells 9(2):266
Khot A, Brajanovski N, Cameron DP, Hein N, Maclachlan KH, Sanij E et al (2019) First-in-human RNA polymerase I transcription inhibitor CX-5461 in patients with advanced hematologic cancers: results of a phase I dose-escalation study. Cancer Discov 9(8):1036–1049
von Walden F, Casagrande V, Ostlund Farrants K, Nader G (2012) Mechanical loading induces the expression of a Pol I regulon at the onset of skeletal muscle hypertrophy. AJP Cell Physiol 302(10):C1523–C1530
Whitelaw PF, Hesketh JE (1992) Expression of c-myc and c-fos in rat skeletal muscle. Evidence for increased levels of c-myc mRNA during hypertrophy. Biochem J 281(Pt 1):143–147
Lai K-MV, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E et al (2004) Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 24(21):9295–9304
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R et al (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3(11):1014–1019
Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E et al (2009) Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J 23(11):3896–3905
Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 169(2):361–371
Goodman CA, Miu MH, Frey JW, Mabrey DM, Lincoln HC, Ge Y et al (2010) A phosphatidylinositol 3-kinase/protein kinase B-independent activation of mammalian target of rapamycin signaling is sufficient to induce skeletal muscle hypertrophy. Mol Biol Cell 21(18):3258–3268
Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL et al (2009) Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587(Pt 7):1535–1546
Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR (2000) Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130(10):2413–2419
Wen Y, Alimov AP, McCarthy JJ (2016) Ribosome biogenesis is necessary for skeletal muscle hypertrophy. Exerc Sport Sci Rev 44(3):110–115
Figueiredo VC (2019) Revisiting the roles of protein synthesis during skeletal muscle hypertrophy induced by exercise. Am J Physiol Regul Integr Comp Physiol 317(5):R709–R718
Nakada S, Ogasawara R, Kawada S, Maekawa T, Ishii N (2016) Correlation between ribosome biogenesis and the magnitude of hypertrophy in overloaded skeletal muscle. PLoS ONE 11(1):e0147284
Figueiredo VC, Englund DA, Vechetti IJ Jr, Alimov A, Peterson CA, McCarthy JJ (2019) Phosphorylation of eukaryotic initiation factor 4E is dispensable for skeletal muscle hypertrophy. Am J Physiol Cell Physiol 317(6):C1247–C1255
Figueiredo VC, Caldow MK, Massie V, Markworth JF, Cameron-Smith D, Blazevich AJ (2015) Ribosome biogenesis adaptation in resistance training-induced human skeletal muscle hypertrophy. Am J Physiol Endocrinol Metab 309(1):E72-83
Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM (2016) Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab 310:E652–E661
Hammarstrom D, Ofsteng S, Koll L, Hanestadhaugen M, Hollan I, Apro W et al (2020) Benefits of higher resistance-training volume are related to ribosome biogenesis. J Physiol 598(3):543–565
Machida M, Takeda K, Yokono H, Ikemune S, Taniguchi Y, Kiyosawa H et al (2012) Reduction of ribosome biogenesis with activation of the mTOR pathway in denervated atrophic muscle. J Cell Physiol 227(4):1569–1576
Connolly M, Paul R, Farre-Garros R, Natanek SA, Bloch S, Lee J et al (2018) miR-424-5p reduces ribosomal RNA and protein synthesis in muscle wasting. J Cachexia Sarcopenia Muscle 9(2):400–416
Figueiredo VC, Markworth JF, Durainayagam BR, Pileggi CA, Roy NC, Barnett MP et al (2016) Impaired ribosome biogenesis and skeletal muscle growth in a murine model of inflammatory bowel disease. Inflamm Bowel Dis 22(2):268–278
Von Walden F, Gantelius S, Liu C, Borgstrom H, Bjork L, Gremark O et al (2018) Muscle contractures in patients with cerebral palsy and acquired brain injury are associated with extracellular matrix expansion, pro-inflammatory gene expression, and reduced rRNA synthesis. Muscle Nerve 58(2):277–285
Fiorotto ML, Davis T, Sosa H, Villegas-Montoya C, Estrada I, Fleischmann R (2014) Ribosome abundance regulates the recovery of skeletal muscle protein mass upon recuperation from postnatal undernutrition in mice. J Physiol 592:5269–5286
Figueiredo VC, D’Souza RF, Van Pelt DW, Lawrence MM, Zeng N, Markworth JF et al (2021) Ribosome biogenesis and degradation regulate translational capacity during muscle disuse and reloading. J Cachexia Sarcopenia Muscle 12(1):130–143
Blagosklonny MV, Pardee AB (2001) Exploiting cancer cell cycling for selective protection of normal cells. Cancer Res 61(11):4301–4305
Minchinton AI, Tannock IF (2006) Drug penetration in solid tumours. Nat Rev Cancer 6(8):583–592
van Asperen J, van Tellingen O, Tijssen F, Schinkel AH, Beijnen JH (1999) Increased accumulation of doxorubicin and doxorubicinol in cardiac tissue of mice lacking mdr1a P-glycoprotein. Br J Cancer 79(1):108–113
Anderson LL, Collins GJ, Ojima Y, Sullivan RD (1970) A study of the distribution of methotrexate in human tissues and tumors. Cancer Res 30(5):1344–1348
Jang MK, Park C, Hong S, Li H, Rhee E, Doorenbos AZ (2020) Skeletal muscle mass change during chemotherapy: a systematic review and meta-analysis. Anticancer Res 40(5):2409–2418
Mijwel S, Cardinale DA, Norrbom J, Chapman M, Ivarsson N, Wengstrom Y et al (2018) Exercise training during chemotherapy preserves skeletal muscle fiber area, capillarization, and mitochondrial content in patients with breast cancer. FASEB J 32(10):5495–5505
Jacobsen PB, Donovan KA, Small BJ, Jim HS, Munster PN, Andrykowski MA (2007) Fatigue after treatment for early stage breast cancer: a controlled comparison. Cancer 110(8):1851–1859
Hydock DS, Lien C-Y, Jensen BT, Schneider CM, Hayward R (2011) Characterization of the effect of in vivo doxorubicin treatment on skeletal muscle function in the rat. Anticancer Res 31(6):2023–2028
Hayward R, Hydock D, Gibson N, Greufe S, Bredahl E, Parry T (2013) Tissue retention of doxorubicin and its effects on cardiac, smooth, and skeletal muscle function. J Physiol Biochem 69(2):177–187
Fabris S, MacLean DA (2015) Skeletal muscle an active compartment in the sequestering and metabolism of doxorubicin chemotherapy. PLoS ONE 10(9):e0139070
de Lima Junior EA, Yamashita AS, Pimentel GD, De Sousa LG, Santos RV, Goncalves CL et al (2016) Doxorubicin caused severe hyperglycaemia and insulin resistance, mediated by inhibition in AMPk signalling in skeletal muscle. J Cachexia Sarcopenia Muscle 7(5):615–625
Nitiss JL (2009) Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer 9(5):338–350
Ray S, Panova T, Miller G, Volkov A, Porter AC, Russell J et al (2013) Topoisomerase IIalpha promotes activation of RNA polymerase I transcription by facilitating pre-initiation complex formation. Nat Commun 4:1598
Guigni BA, Fix DK, Bivona JJ 3rd, Palmer BM, Carson JA, Toth MJ (2019) Electrical stimulation prevents doxorubicin-induced atrophy and mitochondrial loss in cultured myotubes. Am J Physiol Cell Physiol 317(6):C1213–C1228
Bruno PM, Liu Y, Park GY, Murai J, Koch CE, Eisen TJ et al (2017) A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med 23(4):461–471
Guinan EM, Doyle SL, Bennett AE, O’Neill L, Gannon J, Elliott JA et al (2018) Sarcopenia during neoadjuvant therapy for oesophageal cancer: characterising the impact on muscle strength and physical performance. Support Care Cancer 26(5):1569–1576
Donati G, Bertoni S, Brighenti E, Vici M, Treré D, Volarevic S et al (2011) The balance between rRNA and ribosomal protein synthesis up- and downregulates the tumour suppressor p53 in mammalian cells. Oncogene 30(29):3274–3288
Valdez BC, Wang G, Murray D, Nieto Y, Li Y, Shah J et al (2013) Mechanistic studies on the synergistic cytotoxicity of the nucleoside analogs gemcitabine and clofarabine in multiple myeloma: relevance of p53 and its clinical implications. Exp Hematol 41(8):719–730
Wiedenmann B, Malfertheiner P, Friess H, Ritch P, Arseneau J, Mantovani G et al (2008) A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J Support Oncol 6(1):18–25
Jiang HY, Hickey RJ, Abdel-Aziz W, Malkas LH (2000) Effects of gemcitabine and araC on in vitro DNA synthesis mediated by the human breast cell DNA synthesome. Cancer Chemother Pharmacol 45(4):320–328
RuizvanHaperen VW, Veerman G, Vermorken JB, Peters GJ (1993) 2’,2’-Difluoro-deoxycytidine (gemcitabine) incorporation into RNA and DNA of tumour cell lines. Biochem Pharmacol 46(4):762–766
Prado CM, Bekaii-Saab T, Doyle LA, Shrestha S, Ghosh S, Baracos VE et al (2012) Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer 106(10):1583–1586
Quan-Jun Y, Yan H, Yong-Long H, Li-Li W, Jie L, Jin-Lu H et al (2017) Selumetinib attenuates skeletal muscle wasting in murine cachexia model through ERK inhibition and AKT activation. Mol Cancer Ther 16(2):334–343
Prado CM, Purcell SA, Laviano A (2020) Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle 11(2):366–380
Mitchell CJ, Milan AM, Mitchell SM, Zeng N, Ramzan F, Sharma P et al (2017) The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: a 10-wk randomized controlled trial. Am J Clin Nutr 106(6):1375–1383
McKendry J, Thomas ACQ, Phillips SM (2020) Muscle mass loss in the older critically ill population: potential therapeutic strategies. Nutr Clin Pract 35(4):607–616
Figueiredo VC, Zeng N, D’Souza RF, Markworth JF, Della Gatta PA, Petersen A et al (2018) High dose of whey protein after resistance exercise promotes 45 S preribosomal RNA synthesis in older men. Nutrition 50:105–107
Mobley CB, Fox CD, Thompson RM, Healy JC, Santucci V, Kephart WC et al (2015) Comparative effects of whey protein versus L-leucine on skeletal muscle protein synthesis and markers of ribosome biogenesis following resistance exercise. Amino Acids 48(3):733–750
von Walden F, Liu C, Aurigemma N, Nader GA (2016) mTOR signaling regulates myotube hypertrophy by modulating protein synthesis, rDNA transcription and chromatin remodeling. Am J Physiol Cell Physiol 311(4):C663–C672
Haddach M, Schwaebe MK, Michaux J, Nagasawa J, O’Brien SE, Whitten JP et al (2012) Discovery of CX-5461, the first direct and selective inhibitor of RNA polymerase I, for cancer therapeutics. ACS Med Chem Lett 3(7):602–606
Bywater MJ, Poortinga G, Sanij E, Hein N, Peck A, Cullinane C et al (2012) Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell 22(1):51–65
Negi SS, Brown P (2015) rRNA synthesis inhibitor, CX-5461, activates ATM/ATR pathway in acute lymphoblastic leukemia, arrests cells in G2 phase and induces apoptosis. Oncotarget 6(20):18094–18104
Negi SS, Brown P (2015) Transient rRNA synthesis inhibition with CX-5461 is sufficient to elicit growth arrest and cell death in acute lymphoblastic leukemia cells. Oncotarget 6(33):34846–34858
Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J et al (2016) The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med 50(6):339–345
Lahart IM, Metsios GS, Nevill AM, Carmichael AR (2015) Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol (Stockholm, Sweden) 54(5):635–654
Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM (2013) Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol 31(7):876–885
Morikawa A, Naito T, Sugiyama M, Okayama T, Aoyama T, Tanuma A et al (2018) Impact of cancer cachexia on hospitalization-associated physical inactivity in elderly patients with advanced non-small-cell lung cancer. Asia Pac J Oncol Nurs 5(4):377–382
Huneidi SA, Wright NC, Atkinson A, Bhatia S, Singh P (2018) Factors associated with physical inactivity in adult breast cancer survivors—a population-based study. Cancer Med 7(12):6331–6339
Kilroe SP, Fulford J, Holwerda AM, Jackman SR, Lee BP, Gijsen AP et al (2020) Short-term muscle disuse induces a rapid and sustained decline in daily myofibrillar protein synthesis rates. Am J Physiol Endocrinol Metab 318(2):E117–E130
Tyganov SA, Mochalova EP, Belova SP, Sharlo KA, Rozhkov SV, Vilchinskaya NA et al (2019) Effects of plantar mechanical stimulation on anabolic and catabolic signaling in rat postural muscle under short-term simulated gravitational unloading. Front Physiol 10:1252
Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ (2016) Cancer cachexia: beyond weight loss. J Oncol Pract 12(11):1163–1171
Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA (2009) Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 27(17):2758–2765
White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, Henley SJ (2014) Age and cancer risk: a potentially modifiable relationship. Am J Prev Med 46(3 Suppl 1):S7–S15
Evans WJ (2010) Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr 91(4):1123S-S1127
Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R (2017) Prevalence of sarcopenia in the world: a systematic review and meta-analysis of general population studies. J Diab Metab Disord 16:21
Yarasheski KE, Zachwieja JJ, Bier DM (1993) Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol 265(2 Pt 1):E210–E214
Welle S, Thornton C, Statt M (1995) Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am J Physiol 268(3 Pt 1):E422–E427
Volpi E, Mittendorfer B, Wolf SE, Wolfe RR (1999) Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol 277(3):E513–E520
Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A et al (2004) Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 286(3):E321–E328
Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR (2001) Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286(10):1206–1212
Shad BJ, Thompson JL, Breen L (2016) Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am J Physiol Endocrinol Metab 311(5):E803–E817
Campbell WW, Trappe TA, Wolfe RR, Evans WJ (2001) The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci 56(6):M373–M380
English KL, Paddon-Jones D (2010) Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 13(1):34–39
Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, Szewczyk NJ et al (2016) Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol 594(24):7399–7417
Kirby TJ, Lee JD, England JH, Chaillou T, Esser KA, McCarthy JJ (2015) Blunted hypertrophic response in aged skeletal muscle is associated with decreased ribosome biogenesis. J Appl Physiol 1985 119(4):321–327
Stec MJ, Mayhew DL, Bamman MM (2015) The effects of age and resistance loading on skeletal muscle ribosome biogenesis. J Appl Physiol 1985 119(8):851–857
Adamsen L, Quist M, Midtgaard J, Andersen C, Møller T, Knutsen L et al (2006) The effect of a multidimensional exercise intervention on physical capacity, well-being and quality of life in cancer patients undergoing chemotherapy. Support Care Cancer 14(2):116–127
Keogh JWL, MacLeod RD (2012) Body composition, physical fitness, functional performance, quality of life, and fatigue benefits of exercise for prostate cancer patients: a systematic review. J Pain Symptom Manage 43(1):96–110
Mustian KM, Peppone L, Darling TV, Palesh O, Heckler CE, Morrow GR (2009) A 4-week home-based aerobic and resistance exercise program during radiation therapy: a pilot randomized clinical trial. J Support Oncol 7(5):158–167
Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG et al (2003) Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 21(9):1653–1659
Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA (2005) Physical activity and survival after breast cancer diagnosis. JAMA 293(20):2479–2486
Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA et al (2006) Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 24(22):3527–3534
Nader GA, von Walden F, Liu C, Lindvall J, Gutmann L, Pistilli EE et al (2014) Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J Appl Physiol (Bethesda, MD, 1985) 116(6):693–702
Figueiredo VC, Roberts LA, Markworth JF, Barnett MPG, Coombes JS, Raastad T et al (2016) Impact of resistance exercise on ribosome biogenesis is acutely regulated by post-exercise recovery strategies. Physiol Rep 4(2):e12670
Galvão DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, McGuigan MR et al (2006) Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc 38(12):2045–2052
Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM et al (2007) Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol 25(28):4396–4404
Schmitz KH, Ahmed RL, Hannan PJ, Yee D (2005) Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev 14(7):1672–1680
Hanson ED, Wagoner CW, Anderson T, Battaglini CL (2016) The independent effects of strength training in cancer survivors: a systematic review. Curr Oncol Rep 18(5):31
Adamsen L, Quist M, Andersen C, Møller T, Herrstedt J, Kronborg D et al (2009) Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ (Clin Res Ed) 339:b3410
Quist M, Rorth M, Zacho M, Andersen C, Moeller T, Midtgaard J et al (2006) High-intensity resistance and cardiovascular training improve physical capacity in cancer patients undergoing chemotherapy. Scand J Med Sci Sports 16(5):349–357
Brown JC, Schmitz KH (2015) Weight lifting and appendicular skeletal muscle mass among breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat 151(2):385–392
Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU (2010) Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 28(2):340–347
Nilsen TS, Raastad T, Skovlund E, Courneya KS, Langberg CW, Lilleby W et al (2015) Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol (Stockholm, Sweden) 54(10):1805–1813
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM et al (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42(7):1409–1426
Brown JC, Schmitz KH (2014) The prescription or proscription of exercise in colorectal cancer care. Med Sci Sports Exerc 46(12):2202–2209
Jones LW, Eves ND, Peppercorn J (2010) Pre-exercise screening and prescription guidelines for cancer patients. Lancet Oncol 11(10):914–916
Jones LW (2011) Evidence-based risk assessment and recommendations for physical activity clearance: cancer. Appl Physiol Nutri Metabol 36(Suppl 1):S101–S112
Panje CM, Glatzer M, Siren C, Plasswilm L, Putora PM (2018) Treatment options in oncology. JCO Clin Cancer Inform 2:1–10
Funding
The authors have no funding to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Figueiredo, V.C., McCarthy, J.J. Targeting cancer via ribosome biogenesis: the cachexia perspective. Cell. Mol. Life Sci. 78, 5775–5787 (2021). https://doi.org/10.1007/s00018-021-03888-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03888-6