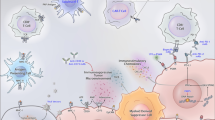
Abstract
Prostate cancer is one of the significant diseases that threaten the survival of men worldwide, with the progression of androgen deprivation therapy, become much rely on it, finally, developed into castration-resistant prostate cancer (ADT). In western countries, ranks second in incidence, and in China, with increasing lifespan, the incidence of prostate cancer is rising steadily. Although chemotherapy agents, such as taxane, have achieved some efficacy, treatment failure still occur. As sensitivity of hormone levels change, the disease can progress to castrate-resistant prostate cancer. Because of the poor efficacy of traditional surgery, endocrine therapy, radiation therapy, and chemotherapy, the treatment options for castrate-resistant prostate cancer are limited. Advanced prostate cancer can progress on immunotherapy, and thus, bio -immunotherapy targeting the unique, prostate microenvironment is an important option. In this paper, we systematically revealed the role of three types of bio-immunotherapies (immune checkpoint inhibitors, tumors, vaccines, cytokines) in castrate-resistant prostate cancer, providing a reference for clinical treatment of prostate cancer.

Similar content being viewed by others
Data availability
In conclusion of all data availability generated or analyzed during this study are included in this published article.
References
Alabi BR, Liu S, Stoyanova T (2022) Current and emerging therapies for neuroendocrine prostate cancer. Pharmacol Ther 238:108255
Ardiani A, Farsaci B, Rogers CJ et al (2013) Combination therapy with a second-generation androgen receptor antagonist and a metastasis vaccine improves survival in a spontaneous prostate cancer model. Clin Cancer Res 19(22):6205–6218
Baeuerle PA, Kufer P, Bargou R (2009) BiTE: teaching antibodies to engage T-cells for cancer therapy. Curr Opin Mol Ther 11(1):22–30
Barqawi AB, Rodrigues Pessoa R, Crawford ED et al (2021) Boosting immune response with GM-CSF optimizes primary cryotherapy outcomes in the treatment of prostate cancer: a prospective randomized clinical trial. Prostate Cancer Prostatic Dis 24(3):750–757
Bonnefoy N, Olive D, Vanhove B (2019) Next generation of anti-immune checkpoints antibodies. Med Sci (paris) 35(12):966–974
Brunet JF, Denizot F, Luciani MF et al (1987) A new member of the immunoglobulin superfamily–CTLA-4. Nature 328(6127):267–270
Carosella ED, Ploussard G, Lemaoult J et al (2015) A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD- L1, and HLA-G. Eur Urol 68(2):267–279
Chen J, Zhang H, Zhou L et al (2020) Enhancing the efficacy of tumor vaccines based on immune evasion mechanisms. Front Oncol 10:584367
Czernin J, Current K, Mona CE et al (2021) Immune-checkpoint blockade enhances (225)Ac-PSMA617 efficacy in a mouse model of prostate cancer. J Nucl Med 62(2):228–231
Deng X, Xiong F, Li X et al (2018) Application of atomic force microscopy in cancer research. J Nanobiotechnol 16(1):102
Deng S, Zhou X, Xu J (2020) Checkpoints under traffic control: from and to organelles. Adv Exp Med Biol 1248:431–453
Dholakia J, Cohen AC, Leath CA 3rd et al (2022) Development of delivery systems for local administration of cytokines/cytokine gene-directed therapeutics: modern implications. Curr Oncol Rep 24(4):389–397
Dranoff G, Jaffee E, Lazenby A et al (1993) Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA 90(8):3539–3543
Fizazi K, Drake CG, Beer TM et al (2020) Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur Urol 78(6):822–830
Graff JN, Liang LW, Kim J et al (2021) KEYNOTE-641: a Phase III study of pembrolizumab plus enzalutamide for metastatic castration-resistant prostate cancer. Future Oncol 17(23):3017–3026
Grenier JM, Yeung ST, Khanna KM (2018) Combination immunotherapy: taking cancer vaccines to the next level. Front Immunol 9:610
Gulley JL, Borre M, Vogelzang NJ et al (2019) Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J Clin Oncol 37(13):1051–1061
Hafron JM, Wilfehrt HM, Ferro C et al (2022) Real-world effectiveness of sipuleucel-T on overall survival in men with advanced prostate cancer treated with androgen receptor-targeting agents. Adv Ther 39(6):2515–2532
Hansen AR, Massard C, Ott PA et al (2018) Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol 29(8):1807–1813
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723
Isaacsson Velho P, Antonarakis ES (2018) PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert Rev Clin Pharmacol 11(5):475–486
Jiang X, Wang J, Deng X et al (2019) Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer 18(1):10
Johnson LE, Frye TP, Arnot AR et al (2006) Safety and immunological efficacy of a prostate cancer plasmid DNA vaccine encoding prostatic acid phosphatase (PAP). Vaccine 24(3):293–303
Junghans RP, Ma QZ, Rathore R et al (2016) Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate 76(14):1257–1270
Kole C, Charalampakis N, Tsakatikas S et al (2020) Immunotherapy for hepatocellular carcinoma: a 2021 update. Cancers (basel) 12(10):2859
Lamont KR, Tindall DJ (2010) Androgen regulation of gene expression. Adv Cancer Res 107:137–162
Lasek W, Zapała Ł (2021) Therapeutic metastatic prostate cancer vaccines: lessons learnt from urologic oncology. Cent European J Urol 74(3):300–307
Lazarus HM, Ragsdale CE, Gale RP et al (2021) Sargramostim (rhu GM-CSF) as cancer therapy (systematic review) and an immunomodulator. A drug before its time? Front Immunol 12:706186
Lin H, Liu Q, Zeng X et al (2021) Pembrolizumab with or without enzalutamide in selected populations of men with previously untreated metastatic castration-resistant prostate cancer harbouring programmed cell death ligand-1 staining: a retrospective study. BMC Cancer 21(1):399
Madan RA, Karzai F, Donahue RN et al (2021) Clinical and immunologic impact of short-course enzalutamide alone and with immunotherapy in non-metastatic castration sensitive prostate cancer. J Immunother Cancer 9(3):e001556
McNeel DG, Eickhoff JC, Johnson LE et al (2019) Phase II trial of a dna vaccine encoding prostatic acid phosphatase (pTVG-HP [MVI-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J Clin Oncol 37(36):3507–3517
Michael BD, Syndikus I, Clark A et al (2010) Diffuse primary leptomeningeal melanocytosis in a patient receiving a novel cancer cell vaccine for prostate cancer. BMJ Case Rep 2010:bcr1120092495
Noguchi M, Fujimoto K, Arai G et al (2021) A randomized phase III trial of personalized peptide vaccination for castration-resistant prostate cancer progressing after docetaxel. Oncol Rep 45(1):159–168
Olson BM, Johnson LE, McNeel DG (2013) The androgen receptor: a biologically relevant vaccine target for the treatment of prostate cancer. Cancer Immunol Immunother 62(3):585–596
Olson BM, Bradley ES, Sawicki T et al (2017) Safety and immunological efficacy of a DNA vaccine encoding the androgen receptor ligand-binding domain (AR-LBD). Prostate 77(7):812–821
Pachynski RK, Morishima C, Szmulewitz R et al (2021) IL-7 expands lymphocyte populations and enhances immune responses to sipuleucel-T in patients with metastatic castration-resistant prostate cancer (mCRPC). J Immunother Cancer 9(8):e002903
Park JH, Rivière I, Gonen M et al (2018) Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 378(5):449–459
Paweletz KL, Li S, Bailis JM et al (2020) Combination of AMG 160, a PSMA x CD3 half-life extended bispecific T-cell engager (HLE BiTE) immune therapy, with an anti-PD-1 antibody in prostate cancer (PCa). J Clin Oncol 38(6):155
Potluri HK, Ng TL, Newton MA et al (2022) GM-CSF elicits antibodies to tumor-associated proteins when used as a prostate cancer vaccine adjuvant. Cancer Immunol Immunother 71(9):2267–2275
Reimers MA, Slane KE, Pachynski RK (2019) Immunotherapy in metastatic castration-resistant prostate cancer: past and future strategies for optimization. Curr Urol Rep 20(10):64
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359(6382):1350–1355
Ross AE, Hurley PJ, Tran PT et al (2020) A pilot trial of pembrolizumab plus prostatic cryotherapy for men with newly diagnosed oligometastatic hormone-sensitive prostate. Prostate Cancer Prostatic Dis 23(1):184–193
Sanda MG, Smith DC, Charles LG et al (1999) Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology 53(2):260–266
Sasada T, Noguchi M, Yamada A et al (2012) Personalized peptide vaccination: a novel immunotherapeutic approach for advanced cancer. Hum Vaccin Immunother 8(9):1309–1313
Schellhammer PF, Chodak G, Whitmore JB et al (2013) Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the immunotherapy for prostate adenocarcinoma treatment (IMPACT) trial. Urology 81(6):1297–1302
Schepisi G, Cursano MC, Casadei C et al (2019) CAR-T cell therapy: a potential new strategy against prostate cancer. J Immunother Cancer. https://doi.org/10.1186/s40425-019-0741-7
Schilling G, Arnold D (2020) Basic principles of immunotherapy. Radiologe 60(8):682–686
Schuster SJ, Bishop MR, Tam CS et al (2019) Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 380(1):45–56
Sharma P, Sohn J, Shin SJ et al (2020) Efficacy and tolerability of tremelimumab in locally advanced or metastatic urothelial carcinoma patients who have failed first- line platinum-based chemotherapy. Clin Cancer Res 26(1):61–70
Shimabukuro-Vornhagen A, Gödel P, Subklewe M et al (2018) Cytokine release syndrome. J Immunother Cancer 6(1):56
孙帅, 朱颐申, 韦萍 (2017) PROSTVAC~□——一种前列腺癌治疗性疫苗的研究进展. 中国疫苗和免疫, 23(01):105–109
Small EJ, Sacks N, Nemunaitis J et al (2007) Granulocyte macrophage colony-stimulating factor–secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res 13(13):3883–3891
Subudhi SK, Siddiqui BA, Aparicio AM et al (2021) Combined CTLA-4 and PD-L1 blockade in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer is associated with increased myeloid and neutrophil immune subsets in the bone microenvironment. J Immunother Cancer 9(10):e002919
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Tarrar TA, Anwar MY, Ali MA et al (2022) Current status of monoclonal antibodies-based therapies in castration-resistant prostate cancer: a systematic review and meta-analysis of clinical trials. Cureus 14(3):e22942
Teo MY, Rathkopf DE, Kantoff P (2019) Treatment of advanced prostate cancer. Annu Rev Med 70:479–499
Thomas S, Prendergast GC (2016) Cancer vaccines: a brief overview. Methods Mol Biol 1403:755–761
van Coillie S, Wiernicki B, Xu J (2020) Molecular and cellular functions of CTLA-4. Adv Exp Med Biol 1248:7–32
Vuky J, Corman JM, Porter C et al (2013) Phase II trial of neoadjuvant docetaxel and CG1940/CG8711 followed by radical prostatectomy in patients with high-risk clinically localized prostate cancer. Oncologist 18(6):687–688
Yerramilli D, Walsh E, Turner E et al (2018) Cancer-related morbidity at the end of life in men with prostate cancer. J Clin Oncol 36(15_suppl):5042
Yu EY, Piulats Rodriguez JMM, Gravis G et al (2020) Pembrolizumab (pembro) plus olaparib in patients (pts) with docetaxel-pretreated metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-365 cohort A efficacy, safety, and biomarker results. American Society of Clinical Oncology
Zuccolotto G, Fracasso G, Merlo A et al (2014) PSMA-specific CAR-engineered T cells eradicate disseminated prostate cancer in preclinical models. PLoS ONE 9(10):e109427
Funding
This work was supported by the Natural Science Foundation of Jiangxi Province (no. 20202BABL206079), funder is WHY.
Author information
Authors and Affiliations
Contributions
LCL conceived the ideas and directed the research; YWH revised the manuscript; CYH, LHR, SLJ, and XHM carried out the scientific literature search, data collection; all authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors all declared no conflicts of interest.
Ethics statement
Approval of the research protocol by an Institutional Reviewer Board: N/A. Informed Consent: N/A. Registry and the Registration No. of the study/trial: N/A. Animal Studies: N/A.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, C., Chen, Y., Shi, L. et al. Advances in bio-immunotherapy for castration-resistant prostate cancer. J Cancer Res Clin Oncol 149, 13451–13458 (2023). https://doi.org/10.1007/s00432-023-05152-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05152-9