Abstract
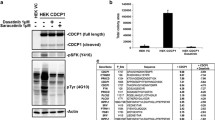
CD24 is a glycosyl-phosphatidylinositol-anchored membrane protein that is frequently over-expressed in a variety of human carcinomas and is correlated with poor prognosis. In cancer cell lines, changes of CD24 expression can alter several cellular properties in vitro and tumor growth in vivo. However, little is known about how CD24 mediates these effects. Here we have analyzed the functional consequences of CD24 knock-down or over-expression in human cancer cell lines. Depletion of CD24 reduced cell proliferation and adhesion, enhanced apoptosis, and regulated the expression of various genes some of which were identified as STAT3 target genes. Loss of CD24 reduced STAT3 and FAK phosphorylation. Diminished STAT3 activity was confirmed by specific reporter assays. We found that reduced STAT3 activity after CD24 knock-down was accompanied by altered Src phosphorylation. Silencing of Src, similar to CD24, targeted the expression of prototype STAT3-regulated genes. Likewise, the over-expression of CD24 augmented Src-Y416 phosphorylation, the recruitment of Src into lipid rafts and the expression of STAT3-dependent target genes. An antibody to CD24 was effective in reducing tumor growth of A549 lung cancer and BxPC3 pancreatic cancer xenografts in mice. Antibody treatment affected the level of Src-phosphorylation in the tumor and altered the expression of STAT3 target genes. Our results provide evidence that CD24 regulates STAT3 and FAK activity and suggest an important role of Src in this process. Finally, the targeting of CD24 by antibodies could represent a novel route for tumor therapy.








Similar content being viewed by others
Abbreviations
- ECM:
-
Extracellular matrix
- FAK:
-
Focal adhesion kinase
- GPI:
-
Glycosyl-phosphatidylinositol
- mAb:
-
Monoclonal antibody
- pAb:
-
Polyclonal antibody
- siCD24:
-
siRNA specific for CD24
- siGFP:
-
siRNA specific for green fluorescent protein (GFP)
- STAT3:
-
Signal transducer and activator of transcription 3
- qPCR:
-
Quantitative real-time PCR
References
Kay R, Rosten PM, Humphries RK (1991) CD24, a signal transducer modulating B cell activation responses, is a very short peptide with a glycosyl phosphatidylinositol membrane anchor. J Immunol 147(4):1412–1416
Kristiansen G, Sammar M, Altevogt P (2004) Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol 35(3):255–262
Kristiansen G, Machado E, Bretz N, Rupp C, Winzer KJ, Konig AK, Moldenhauer G, Marme F, Costa J, Altevogt P (2010) Molecular and clinical dissection of CD24 antibody specificity by a comprehensive comparative analysis. Lab Invest 90(7):1102–1116
Lim SC (2005) CD24 and human carcinoma: tumor biological aspects. Biomed Pharmacother 59(2):S351–S354
Woodward WA, Sulman EP (2008) Cancer stem cells: markers or biomarkers? Cancer Metastasis Rev 27(3):459–470
Fang X, Zheng P, Tang J, Liu Y (2010) CD24: from A to Z. Cell Mol Immunol 7(2):100–103
Nielsen PJ, Lorenz B, Muller AM, Wenger RH, Brombacher F, Simon M, von der Weid T, Langhorne WJ, Mossmann H, Kohler G (1997) Altered erythrocytes and a leaky block in B-cell development in CD24/HSA-deficient mice. Blood 89(3):1058–1067
Hahne M, Wenger RH, Vestweber D, Nielsen PJ (1994) The heat-stable antigen can alter very late antigen 4-mediated adhesion. J Exp Med 179(4):1391–1395
Baumann P, Cremers N, Kroese F, Orend G, Chiquet-Ehrismann R, Uede T, Yagita H, Sleeman JP (2005) CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res 65(23):10783–10793
Sagiv E, Starr A, Rozovski U, Khosravi R, Altevogt P, Wang T, Arber N (2008) Targeting CD24 for treatment of colorectal and pancreatic cancer by monoclonal antibodies or small interfering RNA. Cancer Res 68(8):2803–2812
Taniuchi K, Nishimori I, Hollingsworth MA (2011) Intracellular CD24 inhibits cell invasion by posttranscriptional regulation of BART through interaction with G3BP. Cancer Res 71(3):895–905
Ilangumaran S, Arni S, van Echten-Deckert G, Borisch B, Hoessli DC (1999) Microdomain-dependent regulation of Lck and Fyn protein-tyrosine kinases in T lymphocyte plasma membranes. Mol Biol Cell 10(4):891–905
Zarn JA, Zimmermann SM, Pass MK, Waibel R, Stahel RA (1996) Association of CD24 with the kinase c-fgr in a small cell lung cancer cell line and with the kinase lyn in an erythroleukemia cell line. Biochem Biophys Res Commun 225(2):384–391
Sammar M, Gulbins E, Hilbert K, Lang F, Altevogt P (1997) Mouse CD24 as a signaling molecule for integrin-mediated cell binding: functional and physical association with src-kinases. Biochem Biophys Res Commun 234(2):330–334
Stefanova I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H (1991) GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science 254(5034):1016–1019
Baumann P, Thiele W, Cremers N, Muppala S, Krachulec J, Diefenbacher M, Kassel O, Mudduluru G, Allgayer H, Frame M, Sleeman JP (2012) CD24 interacts with and promotes the activity of Src within lipid rafts in breast cancer cells, thereby increasing integrin-dependent adhesion. Cell Mol Life Sci 69(3):435–448
Bretz N, Noske A, Keller S, Erbe-Hofmann N, Schlange T, Salnikov A, Moldenhauer G, Kristiansen G, Altevogt P (2012) CD24 promotes tumor-cell invasion by suppressing tissue factor pathway inhibitor-2 (TFPI-2) in a Src-dependent fashion. Clin Exp Metast 29(1):27–38
Mierke CT, Bretz N, Altevogt P (2011) Contractile forces contribute to increased GPI-anchored receptor CD24 facilitated cancer cell invasion. J Biol Chem 286(40):34858–34871
Wolterink S, Moldenhauer G, Fogel M, Kiefel H, Pfeifer M, Luttgau S, Gouveia R, Costa J, Endell J, Moebius U, Altevogt P (2010) Therapeutic antibodies to human L1CAM: functional characterization and application in a mouse model for ovarian carcinoma. Cancer Res 70(6):2504–2515
Runz S, Mierke CT, Joumaa S, Behrens J, Fabry B, Altevogt P (2008) CD24 induces localization of beta1 integrin to lipid raft domains. Biochem Biophys Res Commun 365(1):35–41
Jackson D, Waibel R, Weber E, Bell J, Stahel RA (1992) CD24, a signal-transducing molecule expressed on human B cells, is a major surface antigen on small cell lung carcinomas. Cancer Res 52(19):5264–5270
Schabath H, Runz S, Joumaa S, Altevogt P (2006) CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J Cell Sci 119(Pt 2):314–325
Riedle S, Kiefel H, Gast D, Bondong S, Wolterink S, Gutwein P, Altevogt P (2009) Nuclear translocation and signalling of L1-CAM in human carcinoma cells requires ADAM10 and presenilin/gamma-secretase activity. Biochem J 420(3):391–402
Pfeifer M, Schirmer U, Geismann C, Schafer H, Sebens S, Altevogt P (2010) L1CAM expression in endometrial carcinomas is regulated by usage of two different promoter regions. BMC Mol Biol 11:64
Stoeck A, Gast D, Sanderson MP, Issa Y, Gutwein P, Altevogt P (2007) L1-CAM in a membrane-bound or soluble form augments protection from apoptosis in ovarian carcinoma cells. Gynecol Oncol 104(2):461–469
Kiefel H, Bondong S, Erbe-Hoffmann N, Hazin J, Riedle S, Wolf J, Pfeifer M, Arlt A, Schafer H, Muerkoster SS, Altevogt P (2010) L1CAM-integrin interaction induces constitutive NF-kappaB activation in pancreatic adenocarcinoma cells by enhancing IL-1beta expression. Oncogene 29(34):4766–4778
Mechtersheimer S, Gutwein P, Agmon-Levin N, Stoeck A, Oleszewski M, Riedle S, Postina R, Fahrenholz F, Fogel M, Lemmon V, Altevogt P (2001) Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J Cell Biol 155(4):661–673
Smith SC, Oxford G, Wu Z, Nitz MD, Conaway M, Frierson HF, Hampton G, Theodorescu D (2006) The metastasis-associated gene CD24 is regulated by Ral GTPase and is a mediator of cell proliferation and survival in human cancer. Cancer Res 66(4):1917–1922
Senner V, Sturm A, Baur I, Schrell UH, Distel L, Paulus W (1999) CD24 promotes invasion of glioma cells in vivo. J Neuropathol Exp Neurol 58(8):795–802
Dauer DJ, Ferraro B, Song L, Yu B, Mora L, Buettner R, Enkemann S, Jove R, Haura EB (2005) Stat3 regulates genes common to both wound healing and cancer. Oncogene 24(21):3397–3408
Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9(11):798–809
Cao X, Tay A, Guy GR, Tan YH (1996) Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol 16(4):1595–1603
Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R (1998) Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol 18(5):2545–2552
Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT (1994) Autophosphorylation of the focal adhesion kinase, pp 125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol 14(3):1680–1688
Calalb MB, Polte TR, Hanks SK (1995) Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol 15(2):954–963
Hitosugi T, Sato M, Sasaki K, Umezawa Y (2007) Lipid raft specific knockdown of SRC family kinase activity inhibits cell adhesion and cell cycle progression of breast cancer cells. Cancer Res 67(17):8139–8148
Overdevest JB, Thomas S, Kristiansen G, Hansel DE, Smith SC, Theodorescu D (2011) CD24 offers a therapeutic target for control of bladder cancer metastasis based on a requirement for lung colonization. Cancer Res 71(11):3802–3811
Ahmed MA, Jackson D, Seth R, Robins A, Lobo DN, Tomlinson IP, Ilyas M (2009) CD24 is upregulated in inflammatory bowel disease and stimulates cell motility and colony formation. Inflamm Bowel Dis 16(5):795–803
Fukushima T, Tezuka T, Shimomura T, Nakano S, Kataoka H (2007) Silencing of insulin-like growth factor-binding protein-2 in human glioblastoma cells reduces both invasiveness and expression of progression-associated gene CD24. J Biol Chem 282(25):18634–18644
Wang W, Wang X, Peng L, Deng Q, Liang Y, Qing H, Jiang B (2010) CD24-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Cancer Sci 101(1):112–119
Brunton VG, Avizienyte E, Fincham VJ, Serrels B, Metcalf CA 3rd, Sawyer TK, Frame MC (2005) Identification of Src-specific phosphorylation site on focal adhesion kinase: dissection of the role of Src SH2 and catalytic functions and their consequences for tumor cell behavior. Cancer Res 65(4):1335–1342
Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, Solinas G, Karin M (2011) Saturated fatty acids induce Src clustering within membrane subdomains, leading to JNK activation. Cell 147(1):173–184
Rajendran L, Simons K (2005) Lipid rafts and membrane dynamics. J Cell Sci 118(Pt 6):1099–1102
Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1(1):31–39
Paulick MG, Bertozzi CR (2008) The glycosylphosphatidylinositol anchor: a complex membrane-anchoring structure for proteins. Biochemistry 47(27):6991–7000
Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO (2011) CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell 9(1):50–63
Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ, Choudhury SA, Maruyama R, Wu Z, Gonen M, Mulvey LA, Bessarabova MO, Huh SJ, Silver SJ, Kim SY, Park SY, Lee HE, Anderson KS, Richardson AL, Nikolskaya T, Nikolsky Y, Liu XS, Root DE, Hahn WC, Frank DA, Polyak K (2011) The JAK2/STAT3 signaling pathway is required for growth of CD44+ CD24− stem cell-like breast cancer cells in human tumors. J Clin Invest. 121(7):2723–2735
Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, Grabau D, Ferno M, Borg A, Hegardt C (2008) The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res 10(3):R53
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100(7):3983–3988
Acknowledgments
This work was supported by a grant from the DKFZ-BSP Alliance to G. M., T.S. and P. A. We thank Helena Kiefel for valuable comments on the manuscript and Volker Kloess for help and technical support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Fig. 1 Dot-blot analysis of apoptosis after siCD24 knock-down using PI- and Annexin-V staining.
Suppl. Fig. 2 List of genes identified after depletion of CD24 by siCD24-dependent knock-down. SiGFP served as a control.
Suppl. Fig. 3 Western-blot analysis of active (Y416) and inactive (Y527) Src in glioblastoma cell lines after CD24 depletion.
Suppl. Fig. 4 Effect of Src inhibition by the specific inhibitor PP2 on the regulation of STAT3-dependent genes.
Suppl. Fig. 5 (A) Intraperitoneal tumor growth of SKOV3ip ovarian cancer cells in CD1 mice treated with either PBS, isotype control, or SWA11 mAb. Antibodies (10 mg/kg) were given twice a week with eight injections in total. Animals were killed and the tumor mass was determined and used for further analysis. (B) MRNAs were extracted from the isolated tumors and analyzed by RT-PCR for the indicated STAT3-dependent genes. Note that p-Src Y527 staining was undetectable both in SWA11- and isotype-control treated groups.
Rights and permissions
About this article
Cite this article
Bretz, N.P., Salnikov, A.V., Perne, C. et al. CD24 controls Src/STAT3 activity in human tumors. Cell. Mol. Life Sci. 69, 3863–3879 (2012). https://doi.org/10.1007/s00018-012-1055-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1055-9