Abstract
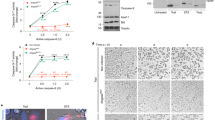
Enterococci are commensal organisms in the alimentary tract. However, they can cause a variety of life-threatening infections, especially in nosocomial settings. We hypothesized that induction of cell death might enable these facultative pathogenic bacteria to evade the innate immune response and to cause infections of their host. We demonstrate that E. faecium when exposed to lysozyme induces cell death in macrophages in vitro and in vivo. Flow cytometric analyses of J774A.1 macrophages infected with E. faecium revealed loss of cell membrane integrity indicated by uptake of propidium iodide and decrease of the inner mitochondrial transmembrane potential ΔΨm. Inhibition of caspases, treatment of macrophages with cytochalasin D, or rifampicin did not prevent cells from dying, suggesting cell death mechanisms that are independent of caspase activation, bacterial uptake, and intracellular bacterial replication. Characteristics of necrotic cell death were demonstrated by both lack of procaspase 3 activation and cell shrinkage, electron microscopy, and release of lactate dehydrogenase. Pretreatment of E. faecium with lysozyme and subsequently with broad spectrum protease considerably reduced cell death, suggesting that a bacterial surface protein is causative for cell death induction. Moreover, in a mouse peritonitis model we demonstrated that E. faecium induces cell death of peritoneal macrophages in vivo. Altogether, our results show that enterococci, under specific conditions such as exposure to lysozyme, induce necrotic cell death in macrophages, which might contribute to disseminated infections by these facultative pathogenic bacteria.






Similar content being viewed by others
References
Jett BD, Huycke MM, Gilmore MS (1994) Virulence of enterococci. Clin Microbiol Rev 7:462–478
Murray BE (1990) The life and times of the Enterococcus. Clin Microbiol Rev 3:46–65
Koch S, Hufnagel M, Theilacker C, Huebner J (2004) Enterococcal infections: host response, therapeutic, and prophylactic possibilities. Vaccine 22:822–830
Chatterjee I, Iredell JR, Woods M, Lipman J (2007) The implications of enterococci for the intensive care unit. Crit Care Resusc 9:69–75
Emori TG, Gaynes RP (1993) An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev 6:428–442
DiazGranados CA, Zimmer SM, Klein M, Jernigan JA (2005) Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis 41:327–333
Ruiz-Garbajosa P, Canton R, Pintado V, Coque TM, Willems R, Baquero F, del Campo R (2006) Genetic and phenotypic differences among Enterococcus faecalis clones from intestinal colonisation and invasive disease. Clin Microbiol Infect 12:1193–1198
Tsuda Y, Shigematsu K, Kobayashi M, Herndon DN, Suzuki F (2008) Role of polymorphonuclear neutrophils on infectious complications stemming from Enterococcus faecalis oral infection in thermally injured mice. J Immunol 180:4133–4138
Miyazaki S, Ohno A, Kobayashi I, Uji T, Yamaguchi K, Goto S (1993) Cytotoxic effect of hemolytic culture supernatant from Enterococcus faecalis on mouse polymorphonuclear neutrophils and macrophages. Microbiol Immunol 37:265–270
Kirschnek S, Scheffel J, Heinzmann U, Hacker G (2004) Necrosis-like cell death induced by bacteria in mouse macrophages. Eur J Immunol 34:1461–1471
Lee W, Lim S, Son HH, Bae KS (2004) Sonicated extract of Enterococcus faecalis induces irreversible cell cycle arrest in phytohemagglutinin-activated human lymphocytes. J Endod 30:209–212
Armeanu S, Krusch M, Baltz KM, Weiss TS, Smirnow I, Steinle A, Lauer UM, Bitzer M, Salih HR (2008) Direct and natural killer cell-mediated antitumor effects of low-dose bortezomib in hepatocellular carcinoma. Clin Cancer Res 14:3520–3528
Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, King KL, Sheehan KC, Yin L, Pennica D, Johnson EM Jr, Schreiber RD (1998) Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 93:373–383
Celada A, Gray PW, Rinderknecht E, Schreiber RD (1984) Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med 160:55–74
Essmann F, Bantel H, Totzke G, Engels IH, Sinha B, Schulze-Osthoff K, Janicke RU (2003) Staphylococcus aureus alpha-toxin-induced cell death: predominant necrosis despite apoptotic caspase activation. Cell Death Differ 10:1260–1272
Krut O, Sommer H, Kronke M (2004) Antibiotic-induced persistence of cytotoxic Staphylococcus aureus in non-phagocytic cells. J Antimicrob Chemother 53:167–173
Haslinger-Loffler B, Kahl BC, Grundmeier M, Strangfeld K, Wagner B, Fischer U, Cheung AL, Peters G, Schulze-Osthoff K, Sinha B (2005) Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell Microbiol 7:1087–1097
Barros LF, Hermosilla T, Castro J (2001) Necrotic volume increase and the early physiology of necrosis. Comp Biochem Physiol A Mol Integr Physiol 130:401–409
Bortner CD, Hughes FM Jr, Cidlowski JA (1997) A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem 272:32436–32442
Carini R, Autelli R, Bellomo G, Albano E (1999) Alterations of cell volume regulation in the development of hepatocyte necrosis. Exp Cell Res 248:280–293
Leendertse M, Willems RJ, Giebelen IA, van den Pangaart PS, Wiersinga WJ, de Vos AF, Florquin S, Bonten MJ, van der Poll T (2008) TLR2-dependent MyD88 signaling contributes to early host defense in murine Enterococcus faecium peritonitis. J Immunol 180:4865–4874
Bantel H, Sinha B, Domschke W, Peters G, Schulze-Osthoff K, Janicke RU (2001) alpha-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J Cell Biol 155:637–648
Timmer AM, Timmer JC, Pence MA, Hsu LC, Ghochani M, Frey TG, Karin M, Salvesen GS, Nizet V (2009) Streptolysin O promotes group A Streptococcus immune evasion by accelerated macrophage apoptosis. J Biol Chem 284:862–871
Genestier AL, Michallet MC, Prevost G, Bellot G, Chalabreysse L, Peyrol S, Thivolet F, Etienne J, Lina G, Vallette FM, Vandenesch F, Genestier L (2005) Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J Clin Invest 115:3117–3127
Jolles P, Jolles J (1984) What’s new in lysozyme research? Always a model system, today as yesterday. Mol Cell Biochem 63:165–189
Gordon S, Todd J, Cohn ZA (1974) In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med 139:1228–1248
Keshav S, Chung P, Milon G, Gordon S (1991) Lysozyme is an inducible marker of macrophage activation in murine tissues as demonstrated by in situ hybridization. J Exp Med 174:1049–1058
Jenzano JW, Hogan SL, Lundblad RL (1986) Factors influencing measurement of human salivary lysozyme in lysoplate and turbidimetric assays. J Clin Microbiol 24:963–967
Fidelman ES, Averyanova LL (1964) Changes in the blood lysozyme concentration in animals following injections of Streptococcus and of homologous tissue antigen. Bull Exp Biol Med 58:39–41
Herbert S, Bera A, Nerz C, Kraus D, Peschel A, Goerke C, Meehl M, Cheung A, Gotz F (2007) Molecular basis of resistance to muramidase and cationic antimicrobial peptide activity of lysozyme in staphylococci. PLoS Pathog 3:e102
Pfeffer JM, Strating H, Weadge JT, Clarke AJ (2006) Peptidoglycan O acetylation and autolysin profile of Enterococcus faecalis in the viable but nonculturable state. J Bacteriol 188:902–908
Hebert L, Courtin P, Torelli R, Sanguinetti M, Chapot-Chartier MP, Auffray Y, Benachour A (2007) Enterococcus faecalis constitutes an unusual bacterial model in lysozyme resistance. Infect Immun 75:5390–5398
Ibrahim HR, Aoki T, Pellegrini A (2002) Strategies for new antimicrobial proteins and peptides: lysozyme and aprotinin as model molecules. Curr Pharm Des 8:671–693
Leendertse M, Willems RJ, Giebelen IA, Roelofs JJ, Bonten MJ, van der Poll T (2009) Neutrophils are essential for rapid clearance of Enterococcus faecium in mice. Infect Immun 77:485–491
Leendertse M, Willems RJ, Giebelen IA, Roelofs JJ, van Rooijen N, Bonten MJ, van der Poll T (2009) Peritoneal macrophages are important for the early containment of Enterococcus faecium peritonitis in mice. Innate Immun 15:3–12
LaFleur AM, Lukacs NW, Kunkel SL, Matsukawa A (2004) Role of CC chemokine CCL6/C10 as a monocyte chemoattractant in a murine acute peritonitis. Mediators Inflamm 13:349–355
Weaver JG, Rouse MS, Steckelberg JM, Badley AD (2004) Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. FASEB J 18:1185–1191
Dozmorov MG, Kyker KD, Saban R, Shankar N, Baghdayan AS, Centola MB, Hurst RE (2007) Systems biology approach for mapping the response of human urothelial cells to infection by Enterococcus faecalis. BMC Bioinformatics 8(Suppl 7):S2
Borgmann S, Schulte B, Wolz C, Gruber H, Werner G, Goerke C, Klare I, Beyser K, Heeg P, Autenrieth IB (2007) Discrimination between epidemic and non-epidemic glycopeptide-resistant E. faecium in a post-outbreak situation. J Hosp Infect 67:49–55
Acknowledgments
The authors thank Birgit Fehrenbacher from the Department of Dermatology and Bettina Hackl from our institute for excellent technical assistance. Additionally, we thank Christopher Weidenmaier from our institute for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gröbner, S., Fritz, E., Schoch, F. et al. Lysozyme activates Enterococcus faecium to induce necrotic cell death in macrophages. Cell. Mol. Life Sci. 67, 3331–3344 (2010). https://doi.org/10.1007/s00018-010-0384-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0384-9