Abstract
Background
Pyroptosis plays a pivotal role in the pathogenesis of many inflammatory diseases. The molecular mechanism by which pyroptosis is induced in macrophages following infection with pathogenic E. coli high pathogenicity island (HPI) will be evaluated in our study.
Results
After infection with the HPI+/HPI− strains and LPS, decreased macrophage cell membrane permeability and integrity were demonstrated with propidium iodide (PI) staining and the lactate dehydrogenase (LDH) assay. HPI+/HPI−-infection was accompanied by upregulated expression levels of NLRP3, ASC, caspase-1, IL-1β, IL-18 and GSDMD, with significantly higher levels detected in the HPI+ group compared to those in the HPI− group (P < 0.01 or P < 0.05). HPI+ strain is more pathogenic than HPI− strain.
Conclusion
Our findings indicate that pathogenic E. coli HPI infection of Saba pigs causes pyroptosis of macrophages characterized by upregulated expression of pyroptosis key factors in the NLRP3/ASC/caspase-1 signaling pathway, direct cell membrane pore formation, and secretion of the inflammatory factor IL-1β and IL-18 downstream of NLRP3 and caspase-1 activation to enhance the inflammatory response.
Similar content being viewed by others
Background
Escherichia coli (E. coli), which is a typical Gram-negative member of the coliform genus Escherichia, plays a key role in the intestinal symbiosis of warm-blooded animals [1, 2]. Pathogenic E. coli strains cause serious harm to human and animal health, often causing severe diarrhea and septicemia [3]. High pathogenicity island (HPI) is a vital factor for the toxicity and pathogenicity of E. coli, and other highly pathogenic strains. It was first discovered in Yersinia [4] and is present only in virulent strains [5]. HPI has a functional core region, containing the irp2-irp1-irp3-irp4-irp5-FyuA gene axis known as the irp2-FyuA gene cluster, and the irp2 marker gene [6]. Paauw et al. demonstrated that Yersinia containing HPI was more virulent by studying the iron carrier encoding HPI [7].
Pyroptosis is a form of programmed cell death (PCD) mediated by gasdermin D (GSDMD), relied on caspase-1, which induces cell swelling and is characterized by the rupture and release of cellular contents leading to an intense inflammatory reaction that is essential for the control of microbial infections [8, 9]. Several characteristics of pyroptosis appear to overlap with apoptosis, although the processes are distinct. Pyroptosis is similar to apoptosis [10] in that the small molecule dye propidium iodide (PI) enters the cell through the interstitial space in the plasma membrane and stains the nucleus [11]. Therefore, the formation of membrane pores in the plasma membrane was identified by PI staining. Pyroptosis is one of the host’s natural immune defense mechanisms against intracellular pathogen infection [12]. Activated caspase-1 and its precursors cleave GSDMD protein induce pyroptosis, and forms specific membrane pores with an inner diameter of 12–14 nm [13]. These pores disrupt the ion concentration gradient across the cell membrane and increase the permeability of the cell membrane, leading to swelling and disintegration of the cells [14,15,16].
NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasomes are mainly composed of intracellular pattern-recognition receptor NLRP3, pre-caspase-1 and apoptosis-associated speck-like protein (ASC) [17], which can be activated by bacteria, viruses, fungi and apoptosis [18], and can also directly stimulate the caspase-1 domain activation. The activated caspase-1 then cleaves inactive pro-interleukin-1β (pro-IL-1β) and pro-interleukin-18 (pro-IL-18) to generate the active pro-inflammatory cytokines IL-1β and IL-18 [19], then released from the cytoplasm through this cell membrane pore [13]. Other immune cells are recruited and stimulated by IL-1β and IL-18, thereby inducing the synthesis of other inflammatory cytokines and enhancing the local and systemic inflammatory response [20].
It has been confirmed that Shigella flexneri, Salmonella, Listeria, Pseudomonas aeruginosa, Francis tularensis, Legionella pneumophila and Yersinia induce caspase-1-dependent pyroptosis in macrophages [20]. During infection, pyroptosis induces the death of host cells, which is an important process by which the growth and reproduction of the pathogenic microorganism is limited and the infection is cleared, thus providing effective protection of the host. Pyroptosis participates in the control of various bacterial infectious diseases. However, its mechanism and regulation mechanism has not been elucidated. Saba pigs are a breed local to Yunnan Province that are reared for their high rate of piglet production and an excellent meat quality. Saba sows are commonly used in hybrid breeding systems in central Yunnan Province [21].
To elucidate the pathogenic mechanism of E. coli, we evaluated the ability of pathogenic E. coli HPI to induce pyroptosis in macrophages, and investigated the underlying mechanism. Hu et al. demonstrated that LPS-activated cells, further stimulated by ATP, induced the activation of inflammatory factors and thereby induced pyroptosis [22]. Therefore, LPS + ATP was used as a positive reference to confirm the occurrence of pyroptosis in our study. In addition, we explored the effects of HPI+ and HPI− strains on host cell infection. This information will provide an in-depth understanding of the mechanism of pyroptosis-related diseases, and highlight new therapeutic targets for clinical treatment of related diseases.
Results
Isolation and identification of pathogenic E. coli HPI
The E. coli HPI strains were cultured for 24 h until bright pink round colonies with smooth and moist surface and flat, neat edges were observed (Fig. 1a). Translucent raised colonies that were round in shape and with a smooth, moist surface were formed on normal nutrient agar medium (Fig. 1b). E. coli were identified as Gram-positive rod-shaped red cells (Fig. 1c). The presence of the irp2 gene in the isolates was determined by PCR amplification using the extracted DNA as a template. In total, 43 of the 96 isolates were irp2 gene positive (44.8%) and 53 were negative (55.2%). Representative results for PCR amplification of the HPI irp2 gene are shown in Fig. 1d.
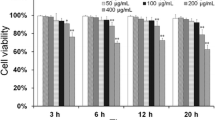
MTT analysis of cell viability
The OD490 of macrophages at 24 h decreased as the concentration of LPS increased. The IC50 for LPS was calculated to be approximately 4.79 ng/mL according to the Improved Kou method (Fig. 2). This concentration of LPS was used in subsequent experiments.
PI staining and concentrations of lactate dehydrogenase (LDH)
The results of PI staining are shown in Fig. 3a. The integrated optical density (IOD) values representing the intensity of PI staining of the HPI groups were significantly higher than that of the control group during the period of 0.5 to 9 h post-infection (P < 0.01). The IOD value of the LPS + ATP group was higher than those in the other groups after infection (P < 0.01). The IOD values in the HPI+ group were markedly higher than those in the HPI− group at different time-points after E. coli infection, with significant differences detected at 0.5 and 9 h (P < 0.05) and extremely significant differences detected at 3 h (P < 0.01) (Fig. 3b). As shown in Fig. 3c, compared to the control group, the LDH concentrations in macrophages in the infection groups were significantly higher during the period of 0.5 to 9 h post-infection (P < 0.01). The LDH contents in the HPI+ group were markedly higher than those in the HPI− group and extremely significantly higher than those detected at 6 h (P < 0.01). After macrophage infection, the IOD values of PI staining and LDH concentrations increased, indicating that HPI caused cell membrane rupture and changes in cell permeability.
a: PI staining of macrophages after E. coli infection (200 × magnification, PI staining, red); b: The IOD value of PI in macrophages at 0.5 h, 3 h and 9 h after E. coli HPI infection. c: The concentration of LDH in macrophages at 0.5, 3, 6 and 9 h after E. coli HPI infection. *P < 0.05, **P < 0.01, n = 3
Effects of HPI infection on the mRNA expression of key pyroptosis genes in macrophages
The NLRP3 inflammasome is composed of NLRP3, ASC and pre-caspase-1. As shown in Fig. 4a, b and c, the relative expression of NLRP3, ASC and caspase-1 mRNA was generally upregulated first and then downregulated in the infection groups. NLRP3, ASC and caspase-1 mRNA expression in the HPI infection groups was significantly higher than that in the control at all time point post-infection (P < 0.01, P < 0.05 or P > 0.05). In addition, there were significant differences in the three genes mRNA expression between the HPI+ and the HPI− groups after infection (P < 0.01 or P < 0.05). At 3 h post-infection, the relative expression of ASC and caspase-1 mRNA in the HPI infection groups was higher than that at the other time-points. As shown in Fig. 4d and e, the expression of IL-1β and IL-18 mRNA in the three infection groups was higher than that in the control group at 6 and 9 h post-infection (P < 0.01), with the highest expression at 9 h post-infection. The IL-1β and IL-18 expression levels in the HPI+ group were significantly different from those in the control and HPI− groups at 6 and 9 h post-infection (P < 0.01). The increased expression levels of these five genes confirmed that HPI induced pyroptosis of macrophages. As shown in Fig. 4f, the expression of GSDMD mRNA in the infection groups was higher than that in the control group, significantly different at 6 and 9 h post-infection (P < 0.01). GSDMD mRNA levels in the HPI+ group were significantly higher than those in the HPI− group at 6 h (P < 0.01). The highest expression of GSDMD at 6 h post-infection indicated that pyroptosis was the most severe at this time.
Immunofluorescence assay (IFA) of NLRP3 and caspase-1 protein expression in macrophages
Immunofluorescence analysis of NLRP3 and caspase-1 expression in macrophages is shown in Fig. 5a and b. The positive cell rate of NLRP3 and caspase-1 in the three infection groups increased first and then decreased during the period from 0.5 to 9 h post-infection (Fig. 5c and d). At all time point post-infection, the positive cell rate of NLRP3 and caspase-1 in the HPI infection groups were significantly higher than those in the control group (P < 0.01). Furthermore, compared with the HPI− group, the NLRP3 positive cell rate in the HPI+ group were significantly higher (P < 0.01) at 0.5, 6 and 9 h after infection, the caspase-1 positive cell rate in the HPI+ group were significantly higher (P < 0.01) at 3 and 6 h after infection.
a: NLRP3 protein in macrophages at 0.5, 3, 6 and 9 h after E. coli HPI infection (NLRP3 staining, red; nuclear staining, blue); b: Caspase-1 protein in macrophages at 0.5, 3, 6 and 9 h after E. coli HPI infection (caspase-1 staining, green; nuclear staining, blue) c: Number of NLRP3 positive cells at 0.5, 3, 6 and 9 h after E. coli HPI infection. d: Number of caspase-1 positive cells at 0.5, 3, 6 and 9 h after E. coli HPI infection. *P < 0.05, **P < 0.01, n = 3
ELISA analysis of IL-1β and IL-18 levels in macrophages after E. coli infection
The concentrations of IL-1β and IL-18 in the culture supernatants of macrophages were detected by ELISA (Fig. 6a and b). The concentrations both cytokines were initially upregulated followed by a gradual decline in expression with time after infection. The IL-1β and IL-18 levels in the LPS + ATP group were significantly higher than those in the control group at 0.5, 3 and 6 h post-infection (P < 0.01). The IL-1β and IL-18 content in the control group was significantly lower than those in the HPI infection groups at 6 and 9 h post-infection (P < 0.01). The concentrations of IL-1β in the HPI+ group were significantly higher than those in the HPI− group at 6 and 9 h post-infection (P < 0.01), the concentrations of IL-18 in the HPI+ group were significantly higher than those in the HPI− group at 0.5, 3 and 9 h post-infection (P < 0.01) and higher than those in the HPI− at the other time-points (P < 0.05).
Discussion
Pyroptosis is a form of innate immune defense against intracellular bacteria [23]. In contrast to apoptosis, numerous pores (1–2 nm) are formed on the cell membrane of pyroptotic cells, leading to the release of the cellular contents and inflammatory factors, such as IL-1β, which further promote the inflammatory response [24]. Studies have shown that when cells undergo pyroptosis, PI can cross the pores in the cell membrane to stain the nucleus red [25]. Fink et al. reported that Salmonella infection of mice caused pyroptosis of host macrophages, with the formation of membrane pores (1.1–2.4 nm) causing cell swelling due to disruption of the osmotic gradient [26]. LDH is an important enzyme in energy metabolism. LDH is released and activity increases as a result of cell death In this study, after infection of macrophages with the pathogenic E. coli HPI, the increase of LDH concentrations in the three experimental groups indicated changes in the membrane permeability. The IOD value of PI staining and the expression of GSDMD mRNA were significantly increased, with higher values in the HPI+ infection group than that in the HPI−. This indicated that E. coli HPI infection promoted the formation of pores in the cell membrane of macrophages, and this effect was more marked in the presence of the HPI irp2 gene. This is consistent with the observation that the number of PI-positive macrophages increased with time after infection with listeria [27].
Muruve and Lamkanfi observed clear signs of pyroptosis and marked increases in the relative mRNA levels of NLRP3, caspase-1, IL-1β and IL-18 after LPS/ATP stimulation of mouse macrophages [28, 29]. Liang reported that the mRNA expression levels of pyroptosis-related genes were increased significantly in renal tissues after obstructive nephropathy caused by unilateral ureteral ligation in rats [30]. In this study, after pathogenic E. coli infection of macrophages in vitro, the intracellular mRNA levels of NLRP3, ASC, caspase-1, IL-1β and IL-18 were higher than those in the control group, with an overall increase in levels observed initially followed by decreased expression, which was consistent with previous reports. The findings indicate that E. coli activates the pyroptosis signaling pathway, and this effect is promoted more effectively by E. coli HPI. In this study, caspase-1 protein expression varied with the different treatments. NLRP3 and caspase-1 protein expression in the LPS + ATP group and the HPI groups increased gradually with time post-infection, which is similar to the pattern of increased caspase-1 protein content in human monocyte macrophages following Helicobacter pylori infection [31]. Furthermore, it has been reported that Neisseria gonorrhoeae infection promoted the activation and secretion of caspase-1 in human monocytes [32]. Our study confirmed that pathogenic E. coli HPI induces pyroptosis in macrophages, and caspase-1 protein expression was higher in host cells infected HPI+ strains containing the HPI irp2 marker gene.
IL-1β and IL-18 play an inflammatory role by binding to the corresponding receptors to regulate the release of soluble antagonists and the expression of precursor enzymes and bait receptors at the transcriptional level. IL-1β and IL-18 are synthesized as inactive cytoplasmic and maturation depends on the caspase-1 activity [33]. The mature forms of IL-1β and IL-18 are released through the GSDMD channel to perform their pro-inflammatory functions and cause pyroptosis [28]. Hitzler showed that H. pylori infection of dendritic cells resulted in activation of caspase-1 and induced the maturation and secretion of IL-1β and IL-18 [34].
The results of our study demonstrated that IL-1β/IL-18 levels were significantly elevated in the culture supernatants of macrophages infected with E.coli HPI, with higher levels detected following infection with the strain carrying the irp2 gene. These findings provide evidence of the initial activation of the NLRP3/caspase-1 pyroptosis pathway in cells following HPI infection, which further promoted the secretion of inflammatory factors IL-1β and IL-18. This is consistent with the significant increase in IL-1β and IL-18 expression after LPS/ATP stimulation of mouse macrophages reported by Wei [35]. These findings effectively confirm that HPI endows characteristics of strong pathogenicity on E. coli, which is closely related to the process of infection.
Conclusion
In conclusion, we found that pathogenic E. coli HPI infection of Yunnan Saba pigs upregulated the expression of NLRP3, caspase-1, IL-1β and IL-18 mRNA, and promoted cell membrane pore formation and nuclear DNA damage in macrophages. Furthermore, we showed that the infection stimulated the release of inflammatory cytokines IL-1β and IL-18, induce inflammation, and eventually promoted pyroptosis of macrophages. Moreover, the existence of HPI in E. coli enhanced the occurrence of pyroptosis of macrophages compared with the effects observed following infection with the HPI− strain. The correlation of E.coli HPI infection with the expression of key pyroptosis-related molecules in monocytes highlights new ideas and directions for further studies to elucidate the molecular mechanism by which E. coli HPI induces pyroptosis in macrophages. Further studies of pyroptosis will contribute to an improved understanding of the mechanisms of cellular injury and the development of pharmaceutical inhibitors of pyroptosis.
Methods
Materials and reagents
The pig macrophage strain 3D4/21 was obtained from BeNa Culture Collection (Beijing). The sampling of E. coli strains was conducted from live Yunnan Saba pigs (a pig farm in Chuxiong City, Yunnan Province, China) through fecal swabs. HPI+ and HPI− strains of pathogenic E. coli were isolated, identified and preserved by the department of animal pathology (Yunnan Agricultural University) [36]. The HPI gene of E.coli was identified by polymerase chain reaction (PCR). HPI+ and HPI− strains have the same serotype (O119) and biochemical characteristics which were tested by the method reported by Jing et al. [37]. Alcohol and chloroform were from Sichuan Xilong Chemical Industry Group Co., Ltd. (Chengdu, China).
PCR detection of HPI irp2 gene and culture of macrophages
The E. coli HPI strains isolated from Saba pigs were cultured on MacConkey agar medium overnight at 37 °C. Individual colonies of pathogenic E. coli were selected, inoculated and cultured on Luria-Bertani (LB) agar plates overnight at 37 °C. At OD600 0.8, bacterial suspensions (containing HPI+/ HPI−), expression of the HPI irp2 genes was analyzed by PCR using the irp2 primers (Table 1). The PCR conditions were as follows: 95 °C 5 min; 94 °C 30 s, 55 °C 30 s, 72 °C 1 min (32 cycles) and one final extension step of 72 °C 8 min. The PCR products were resolved by 1.0% (wt/vol) agarose gel electrophoresis. Macrophages were cultured in DMEM medium containing 10% FBS, 100 U/mL penicillin and streptomycin and incubated at 37 °C under 5% CO2.
MTT assay of cell viability
Macrophages in the control and the experimental groups seeded into 96-well plates at 2 × 105/well with five duplicate wells for each group. Medium (100 μL) containing different concentrations of LPS (106, 105, 104, 103, 102 and 10 ng/mL) was added to each well. After 5.5 h, 10 μL ATP (55 mmol/L) was added. After 24 h, 10 μL MTT reagent (5 mg/mL) was added. After a further 4 h, the culture supernatant was removed and 100 μL DMSO was added. Then absorbance at 490 nm was recorded after 15 min of oscillation. The IC50 for LPS was calculated using the Improved Kou method as follows:
Xm:lg maximum dose; I:lg (maximum dose/ phase dose); P: sum of positive response rates; Pm: maximum positive response rate; Pn: minimum positive response rate.
Macrophages infected with E. coli
The macrophages were randomly divided into four groups: control, HPI+ infection, HPI− infection and LPS + ATP, with three replicates for each group. Macrophages were seeded in 6-well plates at 2 × 106/well, inoculated with HPI+ or HPI− strain at a MOI (multiplicity of infection) of 1 for the indicated time, and LPS + ATP (2 mL LPS at 4.79 ng/mL, 100 μL ATP at 100 mmol/L). At 0.5, 3, 6 and 9 h post-infection, macrophages and their supernatant were collected for analysis.
PI staining and lactate dehydrogenase (LDH) assay
PI staining: After pretreatment, macrophages were seeded in 6-well plates (2 × 106/well) and fixed with 4% paraformaldehyde on ice for 15 min. The cells were cultured with 100 μL of 6.7 μg/mL PI staining solution at 37 °C for 20 min. Macrophages were observed under a light microscope at 20 × objective (Olympus IX73P1F microscope, Japan).
LDH assay: Macrophages were prepared, then the supernatants from cells in each group were collected, the contents of the LDH was detected according to the instructions provided on the kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Quantitative real time PCR (q-PCR)
After pretreatment of macrophages, total RNA was extracted, cDNA was synthesized using q-PCR reverse transcription Kits (TaKaRa, Dalian, China) and stored at − 20 °C. The q-PCR reaction was carried out using gene-specific primers for β-actin, NLRP3, caspase-1, IL-1β and IL-18 (Table 1) with SYBR Premix Ex TaqTM II (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The amplification and detection procedures were carried out using the Bio-Rad Cx96 Detection System (Shanghai, China). The relative mRNA expression level of each index gene was calculated using the 2-△△Ct method.
Immunofluorescence assay (IFA) of HPI on NLRP3 and caspase-1 protein expression into macrophages
After pretreatment, macrophages were seeded in 6-well plates (2× 106/well), fixed, permeabilized and then incubated overnight at 4 °C with anti-NLRP3 (rabbit ployclonal, 1:200, Absin Bioscience Inc., Shanghai, China) and anti-caspase-1 (mouse monoclonal, 1:50, Santa Cruz Biotechnology USA), respectively. Then cells were incubated in the dark with secondary detection antibodies before 4′, 6-diamidino-2-phenylindole (DAPI) treatment. The IOD values for NLRP3 and caspase-1 protein expression were analyzed using Image Pro-Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA). Positive cell rate (%) = Average number of positive cells /Number of DAPI cells × 100.
Elisa
Macrophages were prepared as described in section lactate dehydrogenase (LDH) assay. The supernatants from cells in each group were collected and the contents of the inflammatory mediators IL-1β/IL-18 were detected by commercially available Porcine IL-1β/IL-18 ELISA kits (Shanghai Yuanye Bio-Technology, Co. Ltd., Shanghai, China) according to the manufacturers’ instructions. The coefficient variability of intra-assay and inter-assay was less than 10%.
Statistical analysis
All experimental data were presented as means ± SD (n = 3) with one-way ANOVA, followed by the Duncan post-hoc test used to analyze differences between groups (SPSS, version 19.0, SPSS Science, Chicago, USA). Differences were regarded as significant at P < 0.05, or extremely significant P < 0.01.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- E. coli :
-
Escherichia coli
- HPI:
-
High pathogenicity island
- q-PCR:
-
Quantitative real time PCR
- Ybt:
-
Yersiniabactin
- PCD:
-
Programmed cell death
- GSDMD:
-
Gasdermin D
- PI:
-
Propidium iodide
- IFA:
-
Immunofluorence assay
- LDH:
-
Lactate dehydrogenase
- NLRP3:
-
NOD-like receptor family pyrin domain-containing 3
- ASC:
-
Apoptosis-associated speck-like protein
- CARD:
-
Caspase activation and recruitment domains
- pro-IL-1β:
-
Pro-interleukin-1β
- pro-IL-18:
-
Pro-interleukin-18
References
Stromberg ZR, Johnson JR, Fairbrother JM, Kilbourne J, Van Goor A, Curtiss RR, et al. Evaluation of Escherichia coli isolates from healthy chickens to determine their potential risk to poultry and human health. PLoS One. 2017;12:e0180599.
Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev. 2013;26:822–80.
Makvana S, Krilov LR. Escherichia coli infections. Pediatr Rev. 2015;36:167–71.
Mokracka J, Koczura R, Kaznowski A. Yersiniabactin and other siderophores produced by clinical isolates of Enterobacterspp. And Citrobacterspp. FEMS Immunol Med Microbiol. 2004;40:51–5.
Hacker J, Blum-Oehler G, Muhldorfer I, Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–97.
Schouler C, Schaeffer B, Brée A, Mora A, Dahbi G, Biet F, et al. Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. J Clin Microbiol. 2012;50:1673–8.
Paauw A, Leverstein-van Hall MA, van Kessel KPM, Verhoef J, Fluit AC. Yersiniabactin reduces the respiratory oxidative stress response of innate immune cells. PLoS One. 2009;4:e8240.
Man SM, Kanneganti T-D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16:7–21.
Xia X, Wang X, Zheng Y, Jiang J, Hu J. What role does pyroptosis play in microbial infection? J Cell Physiol. 2018;234:7885–92.
Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–9.
He C, Yang J, Jiang X, Liang X, Lv C. Kaempferol alleviates lps-atp mediated inflammatory injury in splenic lymphocytes via regulation of the pyroptosis pathway in mice. Immunopharmacol Immunotoxicol. 2019;41:1–11.
Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, et al. Caspase-l-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–42.
Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–6.
Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–92.
Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–54.
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–8.
He W-t, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285–98.
Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–86.
Wang S-L, Zhao G, Zhu W, Dong X-M, Liu T, Li Y-Y, et al. Herpes simplex virus-1 infection or simian virus 40-mediated immortalization of corneal cells causes permanent translocation of NLRP3 to the nuclei. Int J Ophthalmol. 2015;8:46.
Lin J, Li D. Pyroptosis: a caspase-1-dependentcelldeath. Int J Immunol. 2011;34:213–6.
Lu S, Lian L. The relationship between serum amylase polymorphism and reproductive performance in Saba pig. Anim Husband Vet Med. 1999:4–6.
Hu Z, Murakami T, Suzuki K, Tamura H, Kuwahara-Arai K, Iba T, et al. Antimicrobial cathelicidin peptide ll-37 inhibits the lps/atp-induced pyroptosis of macrophages by dual mechanism. PLoS One. 2014;9:e85765.
Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–42.
Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8:44–54.
Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology (Baltimore, Md). 2014;59:898–910.
Fink SL, Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol. 2007;9:2562–70.
Cervantes J, Nagata T, Uchijima M, Shibata K, Koide Y. Intracytosolic Listeria monocytogenes induces cell death through caspase-1 activation in murine macrophages. Cell Microbiol. 2008;10:41–52.
Muruve DA, Pétrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–7.
Lamkanfi M, Malireddi RKS, Kanneganti T-D. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 2009;284:20574–81.
Liang W, Ma X, Wang X. Effect of Huayu Jiedu recipe on the expressions of NLRP3, Caspase1, and IL-1β in kidneys of obstructive nephropathy rats. Chin J Integ Trad Western Med. 2017;37:470–5.
Li X, He Y, Liu S. Helicobacter pylori induces cytokines IL-1β and IL-18 production in human monocytic cell line through activation of NLRP3 inflammasome via ROS signalingpathway. Chin J Immunol. 2015;31:308–13.
Ritter JL, Genco CA. Neisseria gonorrhoeae-induced inflammatory Pyroptosis in human macrophages is dependent on intracellular gonococci and Lipooligosaccharide. J Cell Death. 2018;11:1179066017750902.
Hutton HL, Ooi JD, Holdsworth SR, Kitching AR. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology. 2016;21:736–44.
Hitzler I, Sayi A, Kohler E, Engler D, Koch K, Hardt W, et al. Caspase-1 has both proinflammatory and regulatory properties in helicobacter infections, which are differentially mediated by its substrates IL-1β and IL-18. J Immunol. 2012;188:3594–602.
Wei H, Li C, Liang Y. Metformin enhances ATP-stimulated inflammasome activation in LPS-primed peritoneal macrophages. Chin Pharmacol Bull. 2017;33:474–9.
Liu C, Shan C, Dong Q, Fu G, Zhao R, Yan Y, et al. Pathogenic E. coli HPI upregulate the expression of inflammatory factors in porcine small intestinal epithelial cells by ubiquitin proteasome pathway. Res Vet Sci. 2018;120:41–6.
Jing L, Gao F, Shan C. Investigation of HPI gene and drug resistance analysis of piglets Escherichia coli in large-scale pig farms in Chuxiong, Yunnan province. China Anim Husband Vet Med. 2019;46:1849–55.
Acknowledgements
We are grateful to all staff members of College of Animal Science and Technology of Yunnan Agricultural University for their assistance during the experimental work.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31660704; 31960692) and Education Department of Yunnan (Grant No. 2019Y0096). The funders had no role in the study design, data collection and analysis, interpretation of the results, manuscript preparation, or decision to disseminate and publish the study findings.
Author information
Authors and Affiliations
Contributions
CS and SM designed the study and edited the manuscript. WY, HW and JC carried out the experiments. CL, BZ and WZ analyzed the data and prepared all Figures. RZ coordinated all the work. PX and HG responsed for funding acquisition, project administration and supervision. All authors reviewed and approved the final manuscript. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Animals involved in this study were looked after according to the guidelines of Animal Care and Use Committee of Yunnan Agricultural University (NO. YNAU 106760703001). All standard procedures concerning animal care and management were taken throughout the experiment. The written permission from the concerned quarters and the owner(s) of the Saba pigs was obtained prior to sampling and use of animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shan, C., Miao, S., Liu, C. et al. Induction of macrophage pyroptosis-related factors by pathogenic E. coli high pathogenicity island (HPI) in Yunnan Saba pigs. BMC Vet Res 17, 114 (2021). https://doi.org/10.1186/s12917-021-02824-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-021-02824-x