Abstract
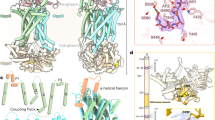
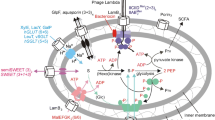
This review focuses on bacterial oligopeptide-binding proteins, which form part of the oligopeptide transport system belonging to the ATP-binding cassette family of transporters. Depending on the bacterial species, these binding proteins (OppA) capture peptides ranging in size from 2 to 18 amino acids from the environment and pass them on to the other components of the oligopeptide transport system for internalisation. Bacteria have developed several strategies to produce these binding proteins, which are periplasmic in Gram– bacteria and membrane-anchored in Gram+, with a higher stoichiometry (probably necessary for efficient transport) than the other components in the transport system. The expression of OppA-encoding genes is clearly modulated by external factors, especially nitrogen compounds, but the mechanisms of regulation are not always clear. The best-understood roles played by OppAs are internalisation of peptides for nutrition and recycling of muropeptides. It has, however, recently become clear that OppAs are also involved in sensing the external medium via specific or non-specific peptides.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Additional information
Received 10 February 2003; received after revision 9 April 2003; accepted 24 April 2003
Rights and permissions
About this article
Cite this article
Monnet, V. Bacterial oligopeptide-binding proteins. CMLS, Cell. Mol. Life Sci. 60, 2100–2114 (2003). https://doi.org/10.1007/s00018-003-3054-3
Issue Date:
DOI: https://doi.org/10.1007/s00018-003-3054-3