Abstract
Objective
Natural killer (NK) cells are part of the innate immune system which not only provides a primary response to pathogenic conditions but can also play an important regulatory role in immune responses. Furthermore, these cells can influence immune responses by affecting other involved cells. Human NK cells can be classified as CD56dim and CD56bright; the former demonstrates mostly cytotoxic effects, while the latter comprises mostly tolerant or regulatory NK cells. These cells participate in the immunopathogenesis of rheumatoid arthritis (RA) and their role remains still unclear.
Methods
We searched PubMed/MEDLINE and Scopus databases to review and analyze relevant literature on the impact of NK cells in the pathogenesis of RA.
Results
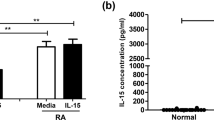
Although the percentage of NK cells increases in peripheral blood of RA patients compared to healthy individuals, the cytotoxic function of these cells is impaired. It is demonstrated by reduced “perforin+ NK cells” and decreased per-cell lytic function. These cytotoxic NK cells may control the pathogenic bone absorptive function of osteoclasts by directly targeting these cells.
Conclusion
Collectively, the evidence collected in the current review emphasizes the possible protective role of CD56dim NK cells in the pathogenesis of RA.

Similar content being viewed by others
References
Chen Z, Bozec A, Ramming A, Schett G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 2019;15(1):9–17. https://doi.org/10.1038/s41584-018-0109-2.
Holers VM, Demoruelle MK, Kuhn KA, Buckner JH, Robinson WH, Okamoto Y, et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol. 2018;14(9):542–57. https://doi.org/10.1038/s41584-018-0070-0.
Jeong HJ, Sohn IW, Kim D, Cho SK, Park SB, Sung IH, et al. Impact of midfoot and Hindfoot involvement on functional disability in Korean patients with rheumatoid arthritis. BMC Musculoskelet Disord. 2017;18(1):365. https://doi.org/10.1186/s12891-017-1726-7.
Ball EM, Bell AL. Lupus arthritis–do we have a clinically useful classification? Rheumatology. 2012;51(5):771–9. https://doi.org/10.1093/rheumatology/ker381.
Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD, Tanasescu R. Extra-articular manifestations in rheumatoid arthritis. Maedica. 2010;5(4):286–91.
Prete M, Racanelli V, Digiglio L, Vacca A, Dammacco F, Perosa F. Extra-articular manifestations of rheumatoid arthritis: an update. Autoimmun Rev. 2011;11(2):123–31. https://doi.org/10.1016/j.autrev.2011.09.001.
Fu B, Tian Z, Wei H. Subsets of human natural killer cells and their regulatory effects. Immunology. 2014;141(4):483–9. https://doi.org/10.1111/imm.12224.
Mandal A, Viswanathan C. Natural killer cells: In health and disease. Hematol Oncol Stem Cell Ther. 2015;8(2):47–55. https://doi.org/10.1016/j.hemonc.2014.11.006.
Karmakar S, Kay J, Gravallese EM. Bone damage in rheumatoid arthritis: mechanistic insights and approaches to prevention. Rheum Dis Clin North Am. 2010;36(2):385–404. https://doi.org/10.1016/j.rdc.2010.03.003.
Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8(11):656–64. https://doi.org/10.1038/nrrheum.2012.153.
Braun T, Zwerina J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res Ther. 2011;13(4):235. https://doi.org/10.1186/ar3380.
Pettit AR, Walsh NC, Manning C, Goldring SR, Gravallese EM. RANKL protein is expressed at the pannus-bone interface at sites of articular bone erosion in rheumatoid arthritis. Rheumatology (Oxford). 2006;45(9):1068–76. https://doi.org/10.1093/rheumatology/kel045.
Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford). 2012;51(Suppl 5):v3-11. https://doi.org/10.1093/rheumatology/kes113.
Mellado M, Martínez-Muñoz L, Cascio G, Lucas P, Pablos JL, Rodríguez-Frade JM. T cell migration in rheumatoid arthritis. Front Immunol. 2015;6:384. https://doi.org/10.3389/fimmu.2015.00384.
Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–12. https://doi.org/10.1084/jem.20071397.
Kosmaczewska A, Swierkot J, Ciszak L, Wiland P. The role of Th1, Th17, and Treg cells in the pathogenesis of rheumatoid arthritis including anti-inflammatory action of Th1 cytokines. Postepy Hig Med Dosw(Online). 2011;65:397–403.
Colin EM, Asmawidjaja PS, van Hamburg JP, Mus AM, van Driel M, Hazes JM, et al. 1,25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010;62(1):132–42. https://doi.org/10.1002/art.25043.
Kunwar S, Dahal K, Sharma S. Anti-IL-17 therapy in treatment of rheumatoid arthritis: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatol Int. 2016;36(8):1065–75. https://doi.org/10.1007/s00296-016-3480-9.
Wei M, Duan D. Efficacy and safety of monoclonal antibodies targeting interleukin-17 pathway for inflammatory arthritis: a meta-analysis of randomized controlled clinical trials. Drug Des Dev Ther. 2016;10:2771–7. https://doi.org/10.2147/dddt.s91374.
Lee YH, Bae SC. Associations between circulating IL-17 levels and rheumatoid arthritis and between IL-17 gene polymorphisms and disease susceptibility: a meta-analysis. Postgrad Med J. 2017;93(1102):465–71. https://doi.org/10.1136/postgradmedj-2016-134637.
Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204(1):41–7. https://doi.org/10.1084/jem.20062259.
Lai Kwan Lam Q, King Hung Ko O, Zheng BJ, Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proceed Natl Acad Sci USA. 2008;105(39):14993–8. https://doi.org/10.1073/pnas.0806044105.
Li J, Hsu HC, Mountz JD. The dynamic duo-inflammatory M1 macrophages and Th17 cells in rheumatic diseases. J Ortho Rheumatol. 2013;1(1):4. https://doi.org/10.13188/2334-2846.1000002.
Agalioti T, Villablanca EJ, Huber S, Gagliani N. T(H)17 cell plasticity: The role of dendritic cells and molecular mechanisms. J Autoimmun. 2018;87:50–60. https://doi.org/10.1016/j.jaut.2017.12.003.
Sun W, Zhang H, Wang H, Chiu YG, Wang M, Ritchlin CT, et al. Targeting notch-activated M1 macrophages attenuates joint tissue damage in a mouse model of inflammatory arthritis. J Bone Mineral Res Off J Am Soc Bone Miner Res. 2017;32(7):1469–80. https://doi.org/10.1002/jbmr.3117.
Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126(4):458–65. https://doi.org/10.1111/j.1365-2567.2008.03027.x.
Yamin R, Berhani O, Peleg H, Aamar S, Stein N, Gamliel M, et al. High percentages and activity of synovial fluid NK cells present in patients with advanced stage active rheumatoid arthritis. Sci Rep. 2019;9(1):1351. https://doi.org/10.1038/s41598-018-37448-z.
Wythe SE, Nicolaidou V, Horwood NJ. Cells of the immune system orchestrate changes in bone cell function. Calcif Tissue Int. 2014;94(1):98–111. https://doi.org/10.1007/s00223-013-9764-0.
Tanaka Y, Maruo A, Fujii K, Nomi M, Nakamura T, Eto S, et al. Intercellular adhesion molecule 1 discriminates functionally different populations of human osteoblasts: characteristic involvement of cell cycle regulators. J Bone Mineral Res Off J Am Soc Bone Miner Res. 2000;15(10):1912–23. https://doi.org/10.1359/jbmr.2000.15.10.1912.
Feng S, Madsen SH, Viller NN, Neutzsky-Wulff AV, Geisler C, Karlsson L, et al. Interleukin-15-activated natural killer cells kill autologous osteoclasts via LFA-1, DNAM-1 and TRAIL, and inhibit osteoclast-mediated bone erosion in vitro. Immunology. 2015;145(3):367–79. https://doi.org/10.1111/imm.12449.
Kurachi T, Morita I, Murota S. Involvement of adhesion molecules LFA-1 and ICAM-1 in osteoclast development. Biochem Biophys Acta. 1993;1178(3):259–66. https://doi.org/10.1016/0167-4889(93)90202-z.
Kakehi S, Nakahama K, Morita I. Expression and possible role of PVR/CD155/Necl-5 in osteoclastogenesis. Mol Cell Biochem. 2007;301(1–2):209–17. https://doi.org/10.1007/s11010-007-9413-x.
Colucci S, Brunetti G, Cantatore FP, Oranger A, Mori G, Pignataro P, et al. The death receptor DR5 is involved in TRAIL-mediated human osteoclast apoptosis. Apoptosis Int J Program Cell Death. 2007;12(9):1623–32. https://doi.org/10.1007/s10495-007-0095-3.
Kovacić N, Lukić IK, Grcević D, Katavić V, Croucher P, Marusić A. The Fas/Fas ligand system inhibits differentiation of murine osteoblasts but has a limited role in osteoblast and osteoclast apoptosis. J Immunol. 2007. https://doi.org/10.4049/jimmunol.178.6.3379.
Song K, Chen Y, Göke R, Wilmen A, Seidel C, Göke A, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J Exp Med. 2000;191(7):1095–104. https://doi.org/10.1084/jem.191.7.1095.
Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408(6812):600–5. https://doi.org/10.1038/35046102.
McInnes IB, al-Mughales J, Field M, Leung BP, Huang FP, Dixon R, et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2(2):175–82. https://doi.org/10.1038/nm0296-175.
Thurkow EW, van der Heijden IM, Breedveld FC, Smeets TJ, Daha MR, Kluin PM, et al. Increased expression of IL-15 in the synovium of patients with rheumatoid arthritis compared with patients with Yersinia-induced arthritis and osteoarthritis. J Pathol. 1997;181(4):444–50. https://doi.org/10.1002/(sici)1096-9896(199704)181:4%3c444::aid-path778%3e3.0.co;2-o.
Andersson AK, Feldmann M, Brennan FM. Neutralizing IL-21 and IL-15 inhibits pro-inflammatory cytokine production in rheumatoid arthritis. Scand J Immunol. 2008;68(1):103–11. https://doi.org/10.1111/j.1365-3083.2008.02118.x.
Ogata Y, Kukita A, Kukita T, Komine M, Miyahara A, Miyazaki S, et al. A novel role of IL 15 in the development of osteoclasts: inability to replace its activity with IL-2. J Immunol. 1999;162(5):2754–60.
Söderström K, Stein E, Colmenero P, Purath U, Müller-Ladner U, de Matos CT, et al. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc Natl Acad Sci USA. 2010;107(29):13028–33. https://doi.org/10.1073/pnas.1000546107.
Takeda H, Kikuchi T, Soboku K, Okabe I, Mizutani H, Mitani A, et al. Effect of IL-15 and natural killer cells on osteoclasts and osteoblasts in a mouse coculture. Inflammation. 2014;37(3):657–69. https://doi.org/10.1007/s10753-013-9782-0.
Pridgeon C, Lennon GP, Pazmany L, Thompson RN, Christmas SE, Moots RJ. Natural killer cells in the synovial fluid of rheumatoid arthritis patients exhibit a CD56bright, CD94bright, CD158negative phenotype. Rheumatology (Oxford). 2003;42(7):870–8. https://doi.org/10.1093/rheumatology/keg240.
de Matos CT, Berg L, Michaëlsson J, Felländer-Tsai L, Kärre K, Söderström K. Activating and inhibitory receptors on synovial fluid natural killer cells of arthritis patients: role of CD94/NKG2A in control of cytokine secretion. Immunology. 2007;122(2):291–301. https://doi.org/10.1111/j.1365-2567.2007.02638.x.
Milush JM, López-Vergès S, York VA, Deeks SG, Martin JN, Hecht FM, et al. CD56negCD16+ NK cells are activated mature NK cells with impaired effector function during HIV-1 infection. Retrovirology. 2013;10:158. https://doi.org/10.1186/1742-4690-10-158.
Voiculescu C, Avramescu C, Balasoiu M, Turculeanu A, Radu E. Changes of blood CD16/CD56 (NK) and HLA-DR/CD3-positive lymphocyte amounts in HIV-infected children, as related to clinical progression and p24-antigen/p24-antibody presence. FEMS Immunol Med Microbiol. 1994;9(3):217–21. https://doi.org/10.1111/j.1574-695X.1994.tb00496.x.
Hu PF, Hultin LE, Hultin P, Hausner MA, Hirji K, Jewett A, et al. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16+CD56+ cells and expansion of a population of CD16dimCD56- cells with low lytic activity. J Acquir Immun Def Syndr Human Retrovirol Off Pub Int Retrovirol Assoc. 1995;10(3):331–40.
Gonzalez VD, Falconer K, Björkström NK, Blom KG, Weiland O, Ljunggren HG, et al. Expansion of functionally skewed CD56-negative NK cells in chronic hepatitis C virus infection: correlation with outcome of pegylated IFN-alpha and ribavirin treatment. J Immunol. 2009. https://doi.org/10.4049/jimmunol.0901437.
Björkström NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208(1):13–21. https://doi.org/10.1084/jem.20100762.
Antonioli CM, Airò P. Dermatomyositis associated with lymphoproliferative disorder of NK cells and occult small cell lung carcinoma. Clin Rheumatol. 2004;23(3):239–41. https://doi.org/10.1007/s10067-003-0814-2.
Nguyen S, Morel V, Le Garff-Tavernier M, Bolgert F, Leblond V, Debré P, et al. Persistence of CD16+/CD56-/2B4+ natural killer cells: a highly dysfunctional NK subset expanded in ocular myasthenia gravis. J Neuroimmunol. 2006;179(1–2):117–25. https://doi.org/10.1016/j.jneuroim.2006.05.028.
Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106(10):3366–9. https://doi.org/10.1182/blood-2005-03-1100.
Barker E, Martinson J, Brooks C, Landay A, Deeks S. Dysfunctional natural killer cells, in vivo, are governed by HIV viremia regardless of whether the infected individual is on antiretroviral therapy. AIDS (London, England). 2007;21(17):2363–5. https://doi.org/10.1097/QAD.0b013e3282f1d658.
Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, et al. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer-cell subsets associated with high levels of HIV-1 viremia. Blood. 2009;114(18):3822–30. https://doi.org/10.1182/blood-2009-06-226332.
Shibatomi K, Ida H, Yamasaki S, Nakashima T, Origuchi T, Kawakami A, et al. A novel role for interleukin-18 in human natural killer cell death: high serum levels and low natural killer cell numbers in patients with systemic autoimmune diseases. Arthritis Rheum. 2001;44(4):884–92. https://doi.org/10.1002/1529-0131(200104)44:4%3c884::aid-anr145%3e3.0.co;2-4.
Dalbeth N, Callan MF. A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum. 2002;46(7):1763–72. https://doi.org/10.1002/art.10410.
Dalbeth N, Gundle R, Davies RJ, Lee YC, McMichael AJ, Callan MF. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol. 2004. https://doi.org/10.4049/jimmunol.173.10.6418.
Shegarfi H, Naddafi F, Mirshafiey A. Natural killer cells and their role in rheumatoid arthritis: friend or foe? The Sci World J. 2012. https://doi.org/10.1100/2012/491974.
Aramaki T, Ida H, Izumi Y, Fujikawa K, Huang M, Arima K, et al. A significantly impaired natural killer cell activity due to a low activity on a per-cell basis in rheumatoid arthritis. Mod Rheumatol. 2009;19(3):245–52. https://doi.org/10.1007/s10165-009-0160-6.
Fathollahi A, Aslani S, Mostafaei S, Rezaei N, Mahmoudi M. The role of killer-cell immunoglobulin-like receptor (KIR) genes in susceptibility to inflammatory bowel disease: systematic review and meta-analysis. Inflamm Res Off J Euro Histamine Res Soc. 2018;67(9):727–36. https://doi.org/10.1007/s00011-018-1162-7.
Horton NC, Mathew PA. NKp44 and natural cytotoxicity receptors as damage-associated molecular pattern recognition receptors. Front Immunol. 2015;6:31. https://doi.org/10.3389/fimmu.2015.00031.
Gunturi A, Berg RE, Forman J. The role of CD94/NKG2 in innate and adaptive immunity. Immunol Res. 2004;30(1):29–34. https://doi.org/10.1385/ir:30:1:029.
Lanier LL. NKG2D receptor and its ligands in host defense. Cancer Immunol Res. 2015;3(6):575–82. https://doi.org/10.1158/2326-6066.cir-15-0098.
Meyaard L. LAIR and collagens in immune regulation. Immunol Lett. 2010;128(1):26–8. https://doi.org/10.1016/j.imlet.2009.09.014.
Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood. 2013;121(18):3599–608. https://doi.org/10.1182/blood-2012-04-425397.
Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10. https://doi.org/10.1038/ni1582.
Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2(10):735–47. https://doi.org/10.1038/nri911.
Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol. 2017;8:1124. https://doi.org/10.3389/fimmu.2017.01124.
Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115(11):2167–76. https://doi.org/10.1182/blood-2009-08-238469.
Della Chiesa M, Marcenaro E, Sivori S, Carlomagno S, Pesce S, Moretta A. Human NK cell response to pathogens. Semin Immunol. 2014;26(2):152–60. https://doi.org/10.1016/j.smim.2014.02.001.
Crouse J, Xu HC, Lang PA, Oxenius A. NK cells regulating T cell responses: mechanisms and outcome. Trends Immunol. 2015;36(1):49–58. https://doi.org/10.1016/j.it.2014.11.001.
Lee SH, Kim KS, Fodil-Cornu N, Vidal SM, Biron CA. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med. 2009;206(10):2235–51. https://doi.org/10.1084/jem.20082387.
Perona-Wright G, Mohrs K, Szaba FM, Kummer LW, Madan R, Karp CL, et al. Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell Host Microbe. 2009;6(6):503–12. https://doi.org/10.1016/j.chom.2009.11.003.
Cook KD, Kline HC, Whitmire JK. NK cells inhibit humoral immunity by reducing the abundance of CD4+ T follicular helper cells during a chronic virus infection. J Leukoc Biol. 2015;98(2):153–62. https://doi.org/10.1189/jlb.4HI1214-594R.
Parisi L, Bassani B, Tremolati M, Gini E, Farronato G, Bruno A. Natural killer cells in the orchestration of chronic inflammatory diseases. J Immunol Res. 2017;2017:4218254. https://doi.org/10.1155/2017/4218254.
Freudenberg J, Gregersen P, Li W. Enrichment of genetic variants for rheumatoid arthritis within T-cell and NK-cell enhancer regions. Mol Med (Cambridge, Mass). 2015;21(1):180–4. https://doi.org/10.2119/molmed.2014.00252.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. https://doi.org/10.1038/nature01658.
Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4(8):638–49. https://doi.org/10.1038/nrg1122.
Firestein GS, Xu WD, Townsend K, Broide D, Alvaro-Gracia J, Glasebrook A, et al. Cytokines in chronic inflammatory arthritis I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J Exp Med. 1988. https://doi.org/10.1084/jem.168.5.1573.
Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345(6274):442–4. https://doi.org/10.1038/345442a0.
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95(7):3597–602. https://doi.org/10.1073/pnas.95.7.3597.
Shigeyama Y, Pap T, Kunzler P, Simmen BR, Gay RE, Gay S. Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum. 2000;43(11):2523–30. https://doi.org/10.1002/1529-0131(200011)43:11%3c2523::aid-anr20%3e3.0.co;2-z.
Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Investig. 2003;111(6):821–31. https://doi.org/10.1172/jci16069.
Li P, Schwarz EM, O’Keefe RJ, Ma L, Boyce BF, Xing L. RANK signaling is not required for TNFalpha-mediated increase in CD11(hi) osteoclast precursors but is essential for mature osteoclast formation in TNFalpha-mediated inflammatory arthritis. J Mineral Res Off J Am Soc Bone Mineral Res. 2004;19(2):207–13. https://doi.org/10.1359/jbmr.0301233.
Zwerina J, Hayer S, Tohidast-Akrad M, Bergmeister H, Redlich K, Feige U, et al. Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum. 2004;50(1):277–90. https://doi.org/10.1002/art.11487.
Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Investig. 2005;115(2):282–90. https://doi.org/10.1172/jci23394.
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–19. https://doi.org/10.1016/s0092-8674(00)80209-3.
Geusens P. The role of RANK ligand/osteoprotegerin in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2012;4(4):225–33. https://doi.org/10.1177/1759720x12438080.
Remuzgo-Martínez S, Genre F, López-Mejías R, Ubilla B, Mijares V, Pina T, et al. Expression of osteoprotegerin and its ligands, RANKL and TRAIL, in rheumatoid arthritis. Sci Rep. 2016;6:29713. https://doi.org/10.1038/srep29713.
Chalan P, Bijzet J, Kroesen BJ, Boots AM, Brouwer E. Altered natural killer cell subsets in seropositive arthralgia and early rheumatoid arthritis are associated with autoantibody status. J Rheumatol. 2016;43(6):1008–16. https://doi.org/10.3899/jrheum.150644.
Lurati A, Bertani L, Marrazza M, Re KA, Bompane D, Scarpellini M. NK cell count as predictor of clinical response in patients with rheumatoid arthritis treated with rituximab. Biol Targets Ther. 2012;6:83–7. https://doi.org/10.2147/btt.s29079.
Daïen CI, Gailhac S, Audo R, Mura T, Hahne M, Combe B, et al. High levels of natural killer cells are associated with response to tocilizumab in patients with severe rheumatoid arthritis. Rheumatology (Oxford). 2015;54(4):601–8. https://doi.org/10.1093/rheumatology/keu363.
Zhu J, Jia E, Zhou Y, Xu J, Feng Z, Wang H, et al. Interleukin-22 secreted by NKp44+ natural killer cells promotes proliferation of fibroblast-like synoviocytes in rheumatoid arthritis. Medicine. 2015;94(52): e2137. https://doi.org/10.1097/md.0000000000002137.
Thanapati S, Ganu M, Giri P, Kulkarni S, Sharma M, Babar P, et al. Impaired NK cell functionality and increased TNF-α production as biomarkers of chronic chikungunya arthritis and rheumatoid arthritis. Hum Immunol. 2017;78(4):370–4. https://doi.org/10.1016/j.humimm.2017.02.006.
Nazari M, Mahmoudi M, Rahmani F, Akhlaghi M, Beigy M, Azarian M, et al. Association of killer cell immunoglobulin- like receptor genes in iranian patients with rheumatoid arthritis. PLoS ONE. 2015;10(12): e0143757. https://doi.org/10.1371/journal.pone.0143757.
Yusa S, Catina TL, Campbell KS. KIR2DL5 can inhibit human NK cell activation via recruitment of Src homology region 2-containing protein tyrosine phosphatase-2 (SHP-2). J Immunol. 2004. https://doi.org/10.4049/jimmunol.172.12.7385.
Acknowledgements
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AF, LN, and MA contributed to the acquisition and interpretation of data and drafting the article. AJ, MM*, and EF* contributed to the interpretation of data, conception and design of the study, and critical revision of the article for important intellectual content. All authors contributed to the revision of the manuscript, read, and gave final approval for the submitted version of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fathollahi, A., Samimi, L.N., Akhlaghi, M. et al. The role of NK cells in rheumatoid arthritis. Inflamm. Res. 70, 1063–1073 (2021). https://doi.org/10.1007/s00011-021-01504-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01504-8