Abstract
Objective and design
We studied the involvement of calcium and calcium-activated NADPH oxidases in NLRP3 inflammasome activation and IL-1β release to better understand inflammasome signaling in macrophages.
Material or subjects
Human volunteer blood donors were recruited to isolate monocytes to differentiate them into macrophages. Wild-type or DUOX1-deficient C57/B6 mice were used to prepare bone marrow-derived macrophages.
Treatment
Murine or human macrophages were treated in vitro with NLRP3 inflammasome agonists (ATP, silica crystals) or calcium agonists (thapsigargin, ionomycin) in calcium-containing or calcium-free medium.
Methods
Intracellular calcium changes were followed by measuring FURA2-based fluorescence. Gene expression changes were measured by quantitative real-time PCR. Protein expression was assessed by western blotting. Enzymatic activity was measured by fluorescence caspase-1 activity assay. IL-1β release was determined by ELISA. ELISA data were analyzed by ANOVA and Tukey’s post hoc test.
Results
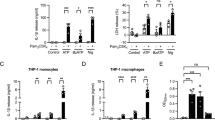
Our data show that calcium is essential for IL-1β release in human macrophages. Increases in cytosolic calcium alone lead to IL-1β secretion. Calcium removal blocks caspase-1 activation. Human macrophages express Duox1, a calcium-regulated NADPH oxidase that produces reactive oxygen species. However, Duox1-deficient murine macrophages show normal IL-1β release.
Conclusions
Human macrophage inflammasome activation and IL-1β secretion requires calcium but does not involve NADPH oxidases.





Similar content being viewed by others
Abbreviations
- BMDM:
-
Bone marrow-derived macrophage
- Duox:
-
Dual oxidase
- DuoxA:
-
Duox Activator
- MDM:
-
Monocyte-derived macrophage
- Nox5:
-
NADPH oxidase 5
- ROS:
-
Reactive oxygen species
References
Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci USA. 2012;109(28):11282–7. doi:10.1073/pnas.1117765109.
Carta S, Tassi S, Pettinati I, Delfino L, Dinarello CA, Rubartelli A. The rate of interleukin-1beta secretion in different myeloid cells varies with the extent of redox response to Toll-like receptor triggering. J Biol Chem. 2011;286(31):27069–80. doi:10.1074/jbc.M110.203398.
Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drobak BK, et al. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989;27(1–2):17–23.
Brayden DJ, Hanley MR, Thastrup O, Cuthbert AW. Thapsigargin, a new calcium-dependent epithelial anion secretagogue. Br J Pharmacol. 1989;98(3):809–16.
Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–26.
Liu C, Hermann TE. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978;253(17):5892–4.
Joosten LA, Ea HK, Netea MG, Busso N. Interleukin-1beta activation during acute joint inflammation: a limited role for the NLRP3 inflammasome in vivo. Joint, Bone, Spine : Revue du Rhumatisme. 2011;78(2):107–10. doi:10.1016/j.jbspin.2010.11.004.
Oosting M, van de Veerdonk FL, Kanneganti TD, Sturm P, Verschueren I, Berende A, et al. Borrelia species induce inflammasome activation and IL-17 production through a caspase-1-dependent mechanism. Eur J Immunol. 2011;41(1):172–81. doi:10.1002/eji.201040385.
Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12(6):593–605. doi:10.1016/j.cmet.2010.11.011.
van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, van der Meer JW, et al. Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci USA. 2010;107(7):3030–3. doi:10.1073/pnas.0914795107.
Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492(7427):123–7. doi:10.1038/nature11588.
Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10(3):210–5. doi:10.1038/nri2725.
Jones JW, Broz P, Monack DM. Innate immune recognition of francisella tularensis: activation of type-I interferons and the inflammasome. Frontiers Microbiol. 2011;2:16. doi:10.3389/fmicb.2011.00016.
Conforti-Andreoni C, Beretta O, Licandro G, Qian HL, Urbano M, Vitulli F, et al. Synergism of NOD2 and NLRP3 activators promotes a unique transcriptional profile in murine dendritic cells. J Leukoc Biol. 2010;88(6):1207–16. doi:10.1189/jlb.1009652.
Mortellaro A, Wong SC, Fric J, Ricciardi-Castagnoli P. The need to identify myeloid dendritic cell progenitors in human blood. Trends Immunol. 2010;31(1):18–23. doi:10.1016/j.it.2009.09.010.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–5. doi:10.1038/nature09663.
Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contribut Microbiol. 2008;15:164–87. doi:10.1159/000136357.
Orient A, Donko A, Szabo A, Leto TL, Geiszt M. Novel sources of reactive oxygen species in the human body. Nephrol, Dialysis, Transpl : Off Pub Eur Dialysis Transpl Assoc Eur Renal Assoc. 2007;22(5):1281–8. doi:10.1093/ndt/gfm077.
Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116(9):1570–3. doi:10.1182/blood-2010-01-264218.
van Bruggen R, Koker MY, Jansen M, van Houdt M, Roos D, Kuijpers TW, et al. Human NLRP3 inflammasome activation is No1–4 independent. Blood. 2010;115(26):5398–400. doi:10.1182/blood-2009-10-250803.
Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, et al. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276(40):37594–601. doi:10.1074/jbc.M103034200.
Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, et al. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem. 2005;280(34):30046–54. doi:10.1074/jbc.M500516200.
Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, Leto TL. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J Off Pub Fed Am Soc Exp Biol. 2009;23(4):1205–18. doi:10.1096/fj.08-120006.
Yoo DG, Winn M, Pang L, Moskowitz SM, Malech HL, Leto TL, et al. Release of cystic fibrosis airway inflammatory markers from Pseudomonas aeruginosa-stimulated human neutrophils involves NADPH oxidase-dependent extracellular DNA trap formation. J Immunol. 2014;192(10):4728–38. doi:10.4049/jimmunol.1301589.
Rada B, Jendrysik MA, Pang L, Hayes CP, Yoo DG, Park JJ, et al. Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PLoS One. 2013;8(1):e54205. doi:10.1371/journal.pone.0054205.
Donko A, Ruisanchez E, Orient A, Enyedi B, Kapui R, Peterfi Z, et al. Urothelial cells produce hydrogen peroxide through the activation of Duox1. Free Radical Biol Med. 2010;49(12):2040–8. doi:10.1016/j.freeradbiomed.2010.09.027.
Rubartelli A, Gattorno M, Netea MG, Dinarello CA. Interplay between redox status and inflammasome activation. Trends Immunol. 2011;32(12):559–66. doi:10.1016/j.it.2011.08.005.
Bauernfeind F, Ablasser A, Bartok E, Kim S, Schmid-Burgk J, Cavlar T, et al. Inflammasomes: current understanding and open questions. Cell Mol Life Sci CMLS. 2011;68(5):765–83. doi:10.1007/s00018-010-0567-4.
Leto TL, Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal. 2006;8(9–10):1549–61. doi:10.1089/ars.2006.8.1549.
Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J Off Pub Fed Am Soc Exp Biol. 2003;17(11):1502–4. doi:10.1096/fj.02-1104fje.
Broz P, Monack DM. Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev. 2011;243(1):174–90. doi:10.1111/j.1600-065X.2011.01041.x.
Conforti-Andreoni C, Ricciardi-Castagnoli P, Mortellaro A. The inflammasomes in health and disease: from genetics to molecular mechanisms of autoinflammation and beyond. Cell Mol Immunol. 2011;8(2):135–45. doi:10.1038/cmi.2010.81.
Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol. 2010;30(5):628–31. doi:10.1007/s10875-010-9440-3.
Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–32. doi:10.1016/j.cell.2010.01.040.
Arlehamn CS, Petrilli V, Gross O, Tschopp J, Evans TJ. The role of potassium in inflammasome activation by bacteria. J Biol Chem. 2010;285(14):10508–18. doi:10.1074/jbc.M109.067298.
Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14(9):1583–9. doi:10.1038/sj.cdd.4402195.
Gao YD, Hanley PJ, Rinne S, Zuzarte M, Daut J. Calcium-activated K(+) channel (K(Ca)3.1) activity during Ca(2+) store depletion and store-operated Ca(2+) entry in human macrophages. Cell Calcium. 2010;48(1):19–27. doi:10.1016/j.ceca.2010.06.002.
Brough D, Le Feuvre RA, Wheeler RD, Solovyova N, Hilfiker S, Rothwell NJ, et al. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J Immunol. 2003;170(6):3029–36.
Choi EM. Regulation of intracellular Ca(2+) by reactive oxygen species in osteoblasts treated with antimycin A. J Appl Toxicol JAT. 2012;32(2):118–25. doi:10.1002/jat.1642.
Rada BK, Geiszt M, Van Bruggen R, Nemet K, Roos D, Ligeti E. Calcium signalling is altered in myeloid cells with a deficiency in NADPH oxidase activity. Clin Exp Immunol. 2003;132(1):53–60.
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–40. doi:10.1038/ni.1831.
Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282(5):2871–9. doi:10.1074/jbc.M608083200.
Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105(26):9035–40. doi:10.1073/pnas.0803933105.
Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–7. doi:10.1126/science.1156995.
Guerra AN, Gavala ML, Chung HS, Bertics PJ. Nucleotide receptor signalling and the generation of reactive oxygen species. Purinergic Signal. 2007;3(1–2):39–51. doi:10.1007/s11302-006-9035-x.
Pfeiffer ZA, Guerra AN, Hill LM, Gavala ML, Prabhu U, Aga M, et al. Nucleotide receptor signaling in murine macrophages is linked to reactive oxygen species generation. Free Radical Biol Med. 2007;42(10):1506–16. doi:10.1016/j.freeradbiomed.2007.02.010.
Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71(2):289–99. doi:10.1016/j.cardiores.2006.05.004.
Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187(2):613–7. doi:10.4049/jimmunol.1100613.
Tassi S, Carta S, Delfino L, Caorsi R, Martini A, Gattorno M, et al. Altered redox state of monocytes from cryopyrin-associated periodic syndromes causes accelerated IL-1beta secretion. Proc Natl Acad Sci USA. 2010;107(21):9789–94. doi:10.1073/pnas.1000779107.
Boots AW, Hristova M, Kasahara DI, Haenen GR, Bast A, van der Vliet A. ATP-mediated activation of the NADPH oxidase DUOX1 mediates airway epithelial responses to bacterial stimuli. J Biol Chem. 2009;284(26):17858–67. doi:10.1074/jbc.M809761200.
Rada B, Lekstrom K, Damian S, Dupuy C, Leto TL. The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol. 2008;181(7):4883–93.
Razzell W, Evans IR, Martin P, Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol CB. 2013;23(5):424–9. doi:10.1016/j.cub.2013.01.058.
Sham D, Wesley UV, Hristova M, van der Vliet A. ATP-mediated transactivation of the epidermal growth factor receptor in airway epithelial cells involves DUOX1-dependent oxidation of Src and ADAM17. PLoS One. 2013;8(1):e54391. doi:10.1371/journal.pone.0054391.
Kwon J, Shatynski KE, Chen H, Morand S, de Deken X, Miot F et al. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Science signaling. 2010;3(133):ra59. doi:10.1126/scisignal.2000976.
Acknowledgments
This study was supported by funds from the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Bernhard Gibbs.
Rights and permissions
About this article
Cite this article
Rada, B., Park, J.J., Sil, P. et al. NLRP3 inflammasome activation and interleukin-1β release in macrophages require calcium but are independent of calcium-activated NADPH oxidases. Inflamm. Res. 63, 821–830 (2014). https://doi.org/10.1007/s00011-014-0756-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-014-0756-y