Abstract
Objective
The aim of this study was to investigate whether the stimulation of monocytic cells with procalcitonin (PCT) results in the release of proinflammatory cytokines. The effects of intravenous immunoglobulin (IVIG) on the production of cytokines from the cells stimulated with PCT were also studied.
Materials and methods
Cultured monocytic cells [THP-1 cells treated with phorbol myristate acetate or peripheral blood mononuclear cells (PBMCs)] were stimulated with PCT. The protein levels of proinflammatory cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and high mobility group box-1] in the culture supernatants were determined by ELISA kits. The concentration of PCT-specific IgG antibody in IVIG was measured using a specific ELISA.
Results
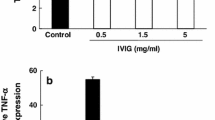
PCT induced the release of cytokines from THP-1 cells in a time- and dose-dependent manner. IVIG inhibited the release of cytokines from the cells stimulated with PCT. It was confirmed that IVIG also inhibited TNF-α release in the same dose range for PBMCs stimulated with PCT. The presence of PCT-specific IgG antibody was detected in the tested IVIG, which might be one of the mechanisms.
Conclusions
PCT induced the release of proinflammatory cytokines from THP-1 cells and PBMCs. The function of PCT was prevented by the presence of IVIG.





Similar content being viewed by others
References
Kreymann KG, de Heer G, Nierhaus A, Kluge S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med. 2007;35:2677–85.
Laupland KB, Kirkpatrick AW, Delaney A. Polyclonal intravenous immunoglobulins for the treatment of severe sepsis and septic shock in critically ill adults: a systematic review and meta-analysis. Crit Care Med. 2007;35:2686–92.
Masaoka T, Hasegawa H, Takaku F, Mizoguchi H, Asano S, Ikeda Y, et al. The efficacy of intravenous immunoglobulin in combination therapy with antibiotics for severe infections. Jpn J Chemother. 2000;48:199–217.
Ulloa L, Tracey KJ. The ‘cytokine profile’: a code for sepsis. Trends Mol Med. 2005;11:56–63.
Yang H, Tracey KJ. Targeting HMGB1 in inflammation. Biochim Biophys Acta. 2010;1799:149–56.
Toungouz M, Denys CH, Groote DD, Dupont E. In vitro inhibition of tumour necrosis factor-α and interleukin-6 production by intravenous immunoglobulins. Br J Haematol. 1995;89:698–703.
Andersson JP, Andersson UG. Human intravenous immunoglobulin modulates monokine production in vitro. Immunology. 1990;71:372–6.
Wu KH, Wu WM, Lu MY, Chiang BL. Inhibitory effect of pooled human immunoglobulin on cytokine production in peripheral blood mononuclear cells. Pediatr Allergy Immunol. 2006;17:60–8.
Becker KL, Nylen ES, White JC, Muller B, Snider RH Jr. Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89:1512–25.
Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49(Suppl 1):S57–61.
Giamarellos-Bourboulis EJ, Mega A, Grecka P, Scarpa N, Koratzanis G, Thomopoulos G, Giamarellou H. Procalcitonin: a marker to clearly differentiate systemic inflammatory response syndrome and sepsis in the critically ill patient? Intensive Care Med. 2002;28:1351–6.
Wiedermann FJ, Kaneider N, Egger P, Tiefenthaler W, Wiedermann CJ, Lindner KH, Schobersberger W. Migration of human monocytes in response to procalcitonin. Crit Care Med. 2002;30:1112–7.
Wei JX, Verity A, Garle M, Mahajan R, Wilson V. Examination of the effect of procalcitonin on human leucocytes and the porcine isolated coronary artery. Br J Anaesth. 2008;100:612–21.
Liappis AP, Gibbs KW, Nylen ES, Yoon B, Snider RH, Gao B, Becker KL. Exogenous procalcitonin evokes a pro-inflammatory cytokine response. Inflamm Res. 2010;60:203–7.
Park EK, Jung HS, Yang HI, Yoo MC, Kim KS. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm Res. 2007;56:45–50.
Pollack M. Antibody activity against Pseudomonas aeruginosa in immune globulins prepared for intravenous use in human. J Infect Dis. 1983;147:1090–8.
Schlievert PM. Use of intravenous immunoglobulin in the treatment of staphylococcal and streptococcal toxin shock syndromes and related illnesses. J Allergy Clin Immunol. 2001;108:S107–10.
Yanagisawa C, Hanaki H, Natae T, Sunakawa K. Neutralization of staphylococcal exotoxins in vitro by human-origin intravenous immunoglobulin. J Infect Chemother. 2007;13:368–72.
Wadhwa M, Meager A, Dilger P, Bird C, Dolman C, Das RG, Thorpe R. Neutralizing antibodies to granulocyte–macrophage colony-stimulating factor, interleukin-1α and interferon-α but not other cytokines in human immunoglobulin preparations. Immunology. 2000;99:113–23.
Nylen ES, Whang KT, Snider RH, Steinwald PM, White JC, Becker KL. Mortality is increased by procalcitonin and decreased by an antiserum reactive to procalcitonin in experimental sepsis. Crit Care Med. 1998;26:1001–6.
Wagner KE, Martinez JM, Vath SD, Snider RH, Nylen ES, Becker KL, Muller B, White JC. Early immunoneutralization of calcitonin precursors attenuates the adverse physiologic response to sepsis in pigs. Crit Care Med. 2002;30:2313–21.
Martinez JM, Wagner KE, Snider RH, Nylen ES, Muller B, Sarani B, Becker KL, White JC. Late immunoneutralization of procalcitonin arrests the progression of lethal porcine sepsis. Surg Infect. 2001;2:193–201. (discussion 202–203).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Artur Bauhofer.
Rights and permissions
About this article
Cite this article
Murakami, K., Suzuki, C., Fujii, A. et al. Intravenous immunoglobulin prevents release of proinflammatory cytokines in human monocytic cells stimulated with procalcitonin. Inflamm. Res. 61, 617–622 (2012). https://doi.org/10.1007/s00011-012-0452-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-012-0452-8