Abstract
Objectives
We have reported that oral erythromycin (EM) inhibits periprosthetic tissue inflammation in a group of patients with aseptic loosening. The purpose of this study was to assess the efficacy of local, periprosthetic EM delivery in a rat model.
Methods
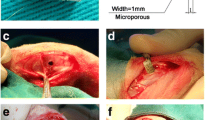
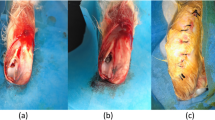
Uncoated Ti pins were press-fit into the right tibia of fourteen Sprague–Dawley rats following an intramedullar injection of UHMWPE (ultra high molecular weight polyethylene) particles. Revision surgeries were performed 2 months after the primary surgery. EM was applied to the Peri-Apatite™ (PA) layer of the titanium (Ti) pins. The previously implanted Ti pins were withdrawn and replaced with Ti pins coated either with (n = 7) or without (n = 7) EM. The rats were killed 1 month after “revision surgery”. The EM efficacy was evaluated by (MicroCT) μCT and histology.
Results
μCT analysis showed that bone volume percentage (BV/TV) was significantly higher in the EM-treated group compared to the untreated group (p < 0.05). Histological analysis showed that EM treatment inhibits UHMWPE particle-induced periprosthetic tissue inflammation compared to the untreated group.
Conclusion
This study demonstrated that periprosthetic EM delivery reduced periprosthetic inflammation and improved the quality of surrounding bone.





Similar content being viewed by others
Abbreviations
- EM:
-
Erythromycin
- PA:
-
Peri-Apatite™
- Ti:
-
Titanium
- S. aureus :
-
Staphylococcus aureus
References
Berry DJ, Harmsen WS, Cabanela ME, Morrey BF. Twenty-five-year survivorship of two thousand consecutive primary charnley total hip replacements : factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002;84:171–7.
Keener JD, Callaghan JJ, Goetz DD, et al. Twenty-five-year results after Charnley total hip arthroplasty in patients less than fifty years old: a concise follow-up of a previous report. J Bone Joint Surg Am. 2003;85-A:1066–72.
Fender D, Harper WM, Gregg PJ. Outcome of Charnley total hip replacement across a single health region in England: the results at five years from a regional hip register [see comments]. J Bone Joint Surg Br. 1999;81:577–81.
Ingham E, Fisher J. Biological reactions to wear debris in total joint replacement. Proc Inst Mech Eng [H]. 2000;214:21–37.
Greenfield EM, Bi Y, Ragab AA, et al. The role of osteoclast differentiation in aseptic loosening. J Orthop Res. 2002;20:1–8.
Merkel KD, Erdmann JM, McHugh KP, et al. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am J Pathol. 1999;154:203–10.
Stea S, Visentin M, Granchi D, et al. Wear debris and cytokine production in the interface membrane of loosened prostheses. J Biomater Sci Polym Ed. 1999;10:247–57.
Maloney WJ, Smith RL, Schmalzried TP, et al. Isolation and characterization of wear particles generated in patients who have had failure of a hip arthroplasty without cement. J Bone Joint Surg Am. 1995;77:1301–10.
Sethi RK, Neavyn MJ, Rubash HE, Shanbhag AS. Macrophage response to cross-linked and conventional UHMWPE. Biomaterials. 2003;24:2561–73.
Wooley PH, Schwarz EM. Aseptic loosening. Gene Ther. 2004;11:402–7.
Schwarz EM. What potential biologic treatments are available for osteolysis? J Am Acad Orthop Surg. 2008;16(Suppl 1):S72–5.
Pollice PF, Rosier RN, Looney RJ, et al. Oral pentoxifylline inhibits release of tumor necrosis factor-alpha from human peripheral blood monocytes: a potential treatment for aseptic loosening of total joint components. J Bone Joint Surg Am. 2001;83-A:1057–61.
Childs LM, Goater JJ, O’Keefe RJ, Schwarz EM. Efficacy of etanercept for wear debris-induced osteolysis. J Bone Miner Res. 2001;16:338–47.
Yatsunami J, Hayashi S. Fourteen-membered ring macrolides as anti-angiogenic compounds. Anticancer Res. 2001;21:4253–8.
Cervin A. The anti-inflammatory effect of erythromycin and its derivatives, with special reference to nasal polyposis and chronic sinusitis. Acta Otolaryngol. 2001;121:83–92.
Giamarellos-Bourboulis EJ. Macrolides beyond the conventional antimicrobials: a class of potent immunomodulators. Int J Antimicrob Agents. 2008;31:12–20.
Nagai H, Shishido H, Yoneda R, et al. Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration. 1991;58:145–9.
Koyama H, Geddes DM. Erythromycin and diffuse panbronchiolitis. Thorax. 1997;52:915–8.
Kudoh S, Azuma A, Yamamoto M, et al. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med. 1998;157:1829–32.
Sakito O, Kadota J, Kohno S, et al. Interleukin 1 beta, tumor necrosis factor alpha, and interleukin 8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: a potential mechanism of macrolide therapy. Respiration. 1996;63:42–8.
Dette GA, Knothe H, Kellner HM. Whole body tissue distribution of [14C]-erythromycin in the guinea pig. an autoradiographic study. Arzneimittelforschung. 1987;37:524–7.
Cuffini AM, Tullio V, Cimino F, Carlone NA. Comparative effects of roxithromycin and erythromycin on cellular immune functions in vitro. 1. Uptake of 3H-macrolides by human macrophages. Microbios. 1989;57:167–78.
Ren WP, Li XY, Chen BD, Wooley PH. Erythromycin inhibits wear debris-induced osteoclastogenesis by modulation of murine macrophage NFkB activity. J Orthop Res. 2004;22:21–9.
Ren WP, Bin W, Mayton L, Wooley PH. Erythromycin (EM) inhibits wear debris-induced inflammatory osteolysis in a murine model. J Orthop Res. 2006;24:280–90.
Ren W, Blasier R, Peng X, et al. Effect of oral erythromycin therapy in patients with aseptic loosening of joint prostheses. Bone. 2009;44:671–7.
Stephenson PK, Freeman MA, Revell PA, et al. The effect of hydroxyapatite coating on ingrowth of bone into cavities in an implant. J Arthroplasty. 1991;6:51–8.
Karabatsos B, Myerthall SL, Fornasier VL, et al. Osseointegration of hydroxyapatite porous-coated femoral implants in a canine model. Clin Orthop Relat Res. 2001;392:442–9.
Hansson U, Ryd L, Toksvig-Larsen S. A randomised RSA study of Peri-Apatite HA coating of a total knee prosthesis. Knee. 2008;15:211–6.
Zhang R, An Y, Toth CA, et al. Osteogenic protein-1 enhances osseointegration of titanium implants coated with peri-apatite in rabbit femoral defect. J Biomed Mater Res B Appl Biomater. 2004;71:408–13.
Zhang R, Xu D, Landeryou T, et al. Ectopic bone formation using osteogenic protein-1 carried by a solution precipitated hydroxyapatite. J Biomed Mater Res A. 2004;71:412–8.
Wooley PH, Morren R, Andary J, et al. Inflammatory responses to orthopaedic biomaterials in the murine air pouch. Biomaterials. 2002;23:517–26.
Ren WP, Yang S, Wooley PH. A novel murine model of orthopaedic wear debris-associated osteolysis. Scand J Rheumatol. 2004;33:349–57.
Allen M, Brett F, Millett P, Rushton N. The effects of particulate polyethylene at a weight-bearing bone-implant interface A study in rats. J Bone Joint Surg Br. 1996;78:32–7.
Morawietz L, Classen RA, Schroder JH, et al. Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J Clin Pathol. 2006;59:591–7.
al Saffar N, Revell PA. Pathology of the bone-implant interfaces. J Long Term Eff Med Implants. 1999;9:319–47.
Jones LC, Frondoza C, Hungerford DS. Immunohistochemical evaluation of interface membranes from failed cemented and uncemented acetabular components. J Biomed Mater Res. 1999;48:889–98.
Yang SY, Ren W, Park Y, et al. Diverse cellular and apoptotic responses to variant shapes of UHMWPE particles in a murine model of inflammation. Biomaterials. 2002;23:3535–43.
Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements as bone drug delivery systems: a review. J Control Release. 2006;113:102–10.
Naber CK. Future strategies for treating Staphylococcus aureus bloodstream infections. Clin Microbiol Infect. 2008;14(Suppl 2):26–34.
Stigter M, Bezemer J, De GK, Layrolle P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J Control Release. 2004;99:127–37.
Stigter M, De GK, Layrolle P. Incorporation of tobramycin into biomimetic hydroxyapatite coating on titanium. Biomaterials. 2002;23:4143–53.
Acknowledgments
This work was supported by an institutional research grant from Stryker Orthopedics (Mahwah, NJ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Michael Parnham.
Rights and permissions
About this article
Cite this article
Ren, W., Zhang, R., Hawkins, M. et al. Efficacy of periprosthetic erythromycin delivery for wear debris-induced inflammation and osteolysis. Inflamm. Res. 59, 1091–1097 (2010). https://doi.org/10.1007/s00011-010-0229-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-010-0229-x