Abstract
Objective and design
In this study, we have investigated the relevance of peripheral blood inflammatory CD14+CD16+ monocytes phenotype to patients with aseptic loosening (AL).
Material and treatment
Immunophenotypes of monocytes were examined among patients with AL (n = 43), patients with mechanical loosening (ML, n = 30), patients with stable implant (SI, n = 16), and patients with osteoarthritis (OA, n = 17) using flow cytometry.
Methods
Immunological assay was used to measure TNF-α and IL-1β levels in both sera and culture media of implant wear stimulated CD14+CD16+ and CD14++CD16− monocytes. Periprosthetic tissues were collected during surgery for histological assessment.
Results
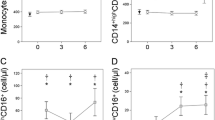
The frequency of CD14+CD16+ monocytes showed significant increase in AL patients than in ML, SI, and OA patients. A positive association was found between the subpopulation of CD14+CD16+ monocytes and plasma TNF-α and IL-1β level in AL patients. Furthermore, a positive correlation existed between the subpopulation of CD14+CD16+ monocytes and the total histopathology score.
Conclusion
The results indicate that CD14+CD16+ monocytes represent a sensitive marker for the disease activity of AL, and may serve as an effective prognostic index to identify total joint replacement recipients who are at increased risk for osteolysis and progression of AL.





Similar content being viewed by others
References
Keener JD, Callaghan JJ, Goetz DD, Pederson DR, Sullivan PM, Johnston RC. Twenty-five-year results after Charnley total hip arthroplasty in patients less than fifty years old: a concise follow-up of a previous report. J Bone Joint Surg Am. 2003;85-A:1066–72.
Sabokbar A, Fujikawa Y, Neale S, Murray DW, Athanasou NA. Human arthroplasty derived macrophages differentiate into osteoclastic bone resorbing cells. Ann Rheum Dis. 1997;56:414–20.
Lee SH, Brennan FR, Jacobs JJ, Urban RM, Ragasa DR, Glant TT. Human monocyte/macrophage response to cobalt-chromium corrosion products and titanium particles in patients with total joint replacements. J Orthop Res. 1997;15:40–9.
Granchi D, Ciapetti G, Stea S, Savarino L, Filippini F, Sudanese A, et al. Cytokine release in mononuclear cells of patients with Co–Cr hip prosthesis. Biomaterials. 1999;20:1079–86.
Matthews JB, Besong AA, Green TR, Stone MH, Wroblewski BM, Fisher J, et al. Evaluation of the response of primary human peripheral blood mononuclear phagocytes to challenge with in vitro generated clinically relevant UHMWPE particles of known size and dose. J Biomed Mater Res. 2000;52:296–307.
Sethi RK, Neavyn MJ, Rubash HE, Shanbhag AS. Macrophage response to cross-linked and conventional UHMWPE. Biomaterials. 2003;24:2561–73.
Wooley PH, Nasser S, Fitzgerald RH Jr. The immune response to implant materials in humans. Clin Orthop Relat Res. 1996;326:63–70.
Wooley PH, Petersen S, Song Z, Nasser S. Cellular immune responses to orthopaedic implant materials following cemented total joint replacement. J Orthop Res. 1997;15:874–80.
Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–34.
Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3.
Unkeless JC. Function and heterogeneity of human Fc receptors for immunoglobulin G. J Clin Invest. 1989;83:355–61.
Ziegler-Heitbrock HW, Fingerle G, Strobel M, Schraut W, Stelter F, Schutt C, et al. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993;23:2053–8.
Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–6.
Skinner NA, MacIsaac CM, Hamilton JA, Visvanathan K. Regulation of Toll-like receptor (TLR)2 and TLR4 on CD14dimCD16 + monocytes in response to sepsis-related antigens. Clin Exp Immunol. 2005;141:270–8.
Skrzeczynska J, Kobylarz K, Hartwich Z, Zembala M, Pryjma J. CD14+ CD16+ monocytes in the course of sepsis in neonates and small children: monitoring and functional studies. Scand J Immunol. 2002;55:629–38.
Kawanaka N, Yamamura M, Aita T, Morita Y, Okamoto A, Kawashima M, et al. CD14+, CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2002;46:2578–86.
Baeten D, Boots AM, Steenbakkers PG, Elewaut D, Bos E, Verheijden GF, et al. Human cartilage gp-39+, CD16+ monocytes in peripheral blood and synovium: correlation with joint destruction in rheumatoid arthritis. Arthritis Rheum. 2000;43:1233–43.
Carracedo J, Merino A, Nogueras S, Carretero D, Berdud I, Ramirez R, et al. On-line hemodiafiltration reduces the proinflammatory CD14+ CD16+ monocyte-derived dendritic cells: a prospective, crossover study. J Am Soc Nephrol. 2006;17:2315–21.
Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–24.
Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–5.
Frankenberger M, Sternsdorf T, Pechumer H, Pforte A, Ziegler-Heitbrock HW. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood. 1996;87:373–7.
Broker BM, Edwards JC, Fanger MW, Lydyard PM. The prevalence and distribution of macrophages bearing Fc gamma R I, Fc gamma R II, and Fc gamma R III in synovium. Scand J Rheumatol. 1990;19:123–35.
Athanasou NA. Synovial macrophages. Ann Rheum Dis. 1995;54:392–4.
Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440.
Maguire JK Jr, Coscia MF, Lynch MH. Foreign body reaction to polymeric debris following total hip arthroplasty. Clin Orthop Relat Res. 1987;216:213–23.
Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, et al. The proinflammatory CD14+ CD16+ DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42.
Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schakel K. The CD16(+) (FcgammaRIII(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J Exp Med. 2002;196:517–27.
Harris WH, McGann WA. Loosening of the femoral component after use of the medullary-plug cementing technique. Follow-up note with a minimum five-year follow-up. J Bone Joint Surg Am. 1986;68:1064–6.
Engh CA, Massin P, Suthers KE. Roentgenographic assessment of the biologic fixation of porous-surfaced femoral components. Clin Orthop Relat Res. 1990;257:107–28.
Mjoberg B, Selvik G, Hansson LI, Rosenqvist R, Onnerfalt R. Mechanical loosening of total hip prostheses. A radiographic and roentgen stereophotogrammetric study. J Bone Joint Surg Br. 1986;68:770–4.
Ryd L, Albrektsson BE, Carlsson L, Dansgard F, Herberts P, Lindstrand A, et al. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg Br. 1995;77:377–83.
Doorn PF, Mirra JM, Campbell PA, Amstutz HC. Tissue reaction to metal on metal total hip prostheses. Clin Orthop Relat Res. 1996;329S:S187–205.
Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C. Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop. 2006;77:177–97.
Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, et al. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001;16:2082–91.
Algan SM, Purdon M, Horowitz SM. Role of tumor necrosis factor alpha in particulate-induced bone resorption. J Orthop Res. 1996;14:30–5.
Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–90.
Hundric-Haspl Z, Pecina M, Haspl M, Tomicic M, Jukic I. Plasma cytokines as markers of aseptic prosthesis loosening. Clin Orthop Relat Res. 2006;453:299–304.
Rader CP, Sterner T, Jakob F, Schutze N, Eulert J. Cytokine response of human macrophage-like cells after contact with polyethylene and pure titanium particles. J Arthroplasty. 1999;14:840–8.
Blumenstein M, Boekstegers P, Fraunberger P, Andreesen R, Ziegler-Heitbrock HW, Fingerle-Rowson G. Cytokine production precedes the expansion of CD14+ CD16+ monocytes in human sepsis: a case report of a patient with self-induced septicemia. Shock. 1997;8:73–5.
Fingerle-Rowson G, Angstwurm M, Andreesen R, Ziegler-Heitbrock HW. Selective depletion of CD14+ CD16+ monocytes by glucocorticoid therapy. Clin Exp Immunol. 1998;112:501–6.
Acknowledgments
This work was supported by Program for Shanghai Key Laboratory of Orthopaedic Implant (08DZ2230330). We would like to thank Dr. John Cuckler, University of Alabama, Birmingham, AL for providing us UHMWPE particles. Thanks also to Dr. Peng Xiaochun for his kind help during patients recruitment and clinical specimen retrieval. And to Prof. Wang Lizhen and Prof. Li Jiang for their kind help in histological assessment of tissue sections.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: J. A. Di Battista.
Rights and permissions
About this article
Cite this article
Wu, W., Zhang, X., Zhang, C. et al. Expansion of CD14+CD16+ peripheral monocytes among patients with aseptic loosening. Inflamm. Res. 58, 561–570 (2009). https://doi.org/10.1007/s00011-009-0020-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-009-0020-z