Abstract
Information on the pyrolysis degradation kinetics of feedstock is critical for the design and fabrication of pyrolysis reactors. In this study, the pyrolytic behaviour of a novel biomass, camel manure, has been studied in a non-isothermal thermogravimetric analyser. The thermal analyses were performed from room temperature to 950 °C at different heating rates (10, 20, and 50°C/min) in an N2 environment (100 ml/min). The kinetic parameters of the manure components are determined using an Arrhenius model equation, and the influence of heating rate on the pyrolytic characteristics and kinetic parameters of the manure components has been examined. The results indicated that the increase in heating rates increased the starting and ending temperatures (decomposition) of manure components. The increase in heating rates shifted the ignition, peak, and burnout temperatures to higher temperatures. At the same time, the increase in heating rates improved the char yield from 22.50 to 31.51%. However, the increase in heating rates showed an insignificant effect on the kinetic parameters of the camel manure components.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Camels are of societal and economic importance to the people of Qatar and the Middle East. The country has been traditionally known for its bondage to the animal. Camels in this part of the world are one-humped dromedary (Camelus dromedarius) species, and they have been historically tamed as a means of transport and also for their meat and milk. Currently, there are about 126,000 camels in Qatar alone [1]. A well-grown camel excretes around 0.95 T of dung every year [2]. This means that the country is producing 120,000 T of camel manure annually [3].
Previous literature studies indicate that the camel manure can be used as a fuel (without any pre-treatment) and fertilizer. However, the usage of the manure for the aforementioned applications is very limited particularly in Qatar, considering its available quantity [4, 5]. Rather, the dung is often landfilled as waste, which is prone to emanate a foul smell causing potential health and environmental issues. Hence, the present study attempts to explore an appropriate method to make use of the available manure and to valorise the manure in a secured way without adversely affecting the people and the environment.
As the dung is a zero-cost feedstock, its application for the recovery of energy and chemicals is attracting more attention [6]. Commonly, several livestock manures are treated using both thermochemical and biochemical methods such as pyrolysis, hydrothermal liquefaction, gasification, composting, aerobic digestion, and anaerobic digestion to extract value-added products from them. The methods are often chosen based on the nature of the dung, demand for products, and availability of resources. Relative to biochemical methods, thermochemical methods are advantageous because they can handle lignocellulosic feedstocks, such as animal manures more efficiently [7]. Furthermore, the process operating parameters of the thermochemical processes can be easily controlled/adjusted and the intended product can be produced straightforwardly. The thermochemical routes also require significantly less water for their operation [8]. Among the thermochemical treatment methods, pyrolysis looks more fascinating as it can deliver an array of products such as char, bio-oil, and syngas [9, 10]. The char finds it application as a soil conditioner, fuel, and adsorbent [11]. The bio-oil is used as a fuel and lubricant, while the syngas is utilised as a fuel and chemical feedstock and also to generate power. Among the pyrolysis products, biochar has a huge demand globally due to its growing widespread use as fertilizer and soil amendment agent [12, 13]. Biochar renders plentiful benefits to plants and soil, thus enriching soil fertility and improving plants yield greatly [14]. Pyrolysis is the most convenient method to generate biochar from any carbonaceous material.
Pyrolysis is the process of converting an organic-rich feedstock into a more useful product in an oxygen-starved atmosphere [15]. The process involves a series of many complicated reactions. Due to this, the understanding of the pyrolytic behaviour of feedstock is indispensable for the layout design and manufacture of pyrolysers and their associated accessories [16]. Thermogravimetric analysis (TGA) is a technique that is widely employed to comprehend the thermal and kinetic behaviour of feedstock [17, 18]. The technique provides data on weight loss of samples (under study) corresponding to the change with respect to time and temperature. The thermogravimetry (TG) and derivative thermogravimetry (DTG) derived from the analysis data are applied to predict the thermal degradation characteristics of samples [19].
Plant biomass is mainly constituted of moisture, cellulose, hemicellulose, lignin, extractives, and ash (inorganics) [20]. The animals or livestock that feed on only plants/trees are called herbivores. Therefore, the manure of the herbivore livestock also has similar constituents as plant biomass. The composition of pyrolysis products mainly depends on the extent of decomposition of lignocellulosic components [21]. The decomposition of hemicellulose and cellulose (holocellulose) produces more volatile products, while the degradation of lignin favours char generation. Among the lignocellulosic components, hemicellulose readily undergoes decomposition, while lignin goes through its degradation phase very slowly. In fact, the degradation of lignin determines the rate of the pyrolysis process. When biomass decomposes, dehydration happens first at temperatures below 150 °C followed by hemicellulose decomposition occurring below 350 °C. The cellulose degrades soon after hemicellulose degradation between 250 and 500 °C which is then followed by lignin decomposition taking place above 400 °C [22].
The information on the thermal behaviour of feedstock during pyrolysis is critical in order to understand the degradation temperature ranges of biomass components and also to assign temperatures for real-time pyrolysis experiments. The study of the kinetics of the pyrolytic process is essential for the design of pyrolysers and also for the optimisation of process parameters [23]. The findings of the study can also be used for process modelling and simulation studies [24]. The kinetic study involves determining three parameters—the pre-exponential factor (frequency factor), the order of reaction, and the activation energy. The pre-exponential factor is the number of collisions of molecules in a unit time taking place during a reaction. The activation energy is defined as the quantity of energy, which is required for a reactant to undergo a reaction. The order of reaction is a function of the speed at which a reaction is taking place. These kinetic parameters are strongly dependent on the nature of sample, particle size, the shape of sample particles, the carrier gas, and the heating rate and also on the mathematical model that is employed to determine the kinetics of the process.
The pyrolytic and kinetic behaviour of many livestock manures such as cattle, goat, horse, swine, and chicken have been studied so far. For example, the pyrolytic characteristics of cattle manure using TGA was studied by Ngo et al. [25], the pyrolytic property of goat manure was investigated by Touray et al. [26], the pyrolytic behaviour of horse manure was examined by Chong et al. [27], while the pyrolytic responses of swine and chicken manure were explored by Tu et al. [28] . However, the investigation of the pyrolysis characteristics of camel manure using TGA, which has been performed in this study, is quite novel. There are many studies available discussing various thermal models such as Kissinger, Friedman, Starink, Vyazovkin, Kissinger-Akahira-Sunose (KAS), Coats-Redfern (CR), Flynn-Wall-Ozawa (FWO), and Discrete Distributed Activation energy model (DAEM) [29]. However, the kinetic studies employing the Arrhenius model/linear multiple regression are very limited. The advantage of the Arrhenius model is that it only needs single heating rate data to determine the kinetics of the thermal decomposition, whereas the other models (FWO, KAS, DAEM, etc.) require multiple heating rates [30]. Furthermore, in the Arrhenius model, the order of the reaction is determined as such and it is not assumed to be one as in the CR model [31]. Hence, in this study, the Arrhenius model has been employed to investigate the kinetics of the camel manure pyrolysis. As discussed earlier, the kinetic parameters are influenced by the pyrolysis process parameters such as heating rate, atmosphere, and particle size of sample. The influence of the above conditions on the kinetics of camel manure pyrolysis has not been explored so far. Keeping the above literature gap in mind, it has been proposed to focus on the following objectives: (i) to provide a basic and fundamental insight into the pyrolytic behaviour of camel manure and (ii) to investigate the influence of heating rate on the pyrolytic characteristics and kinetic factors of camel manure components. The main novelty of the work is the consideration of camel manure as a feedstock for pyrolysis. The study on the effect of heating rate on the pyrolytic behaviour of came manure is also quite new. A logic diagram illustrating the methodology of the current study is presented in Fig. 1.
2 Materials and methods
The camel manure was procured from a local farm. The photograph of the fresh manure is presented in Fig. 2. The manure, as such, had an initial moisture content of 38.00%. However, the feedstock for pyrolysis should preferably have a moisture content below 15%, thus reducing the high quality (high temperature) requirement [32, 33]. Hence, the manure sample was dried under the sun for several days. On each day, the sample was tested for its moisture content using a hot air oven. After the 7th day, the sample displayed a moisture content of 12%. This was also confirmed using proximate analysis, and the dried manure was then subjected to proximate, ultimate, and TG analyses.
The proximate analysis was conducted using a SDT 650 thermogravimetry analyser. The ASTM D7582-12 method was followed for determining the proximate analysis. The elemental/ultimate analysis of the manure was performed in a Euro-vector Euro EA 3000 CHN elemental analyser (EUROVECTOR Srl, Pavia, Italy). The analysis was carried out according to the ASTM D 3176–8. The HHV of the manure was calculated from the proximate analysis results using the below empirical equation [34].
where V corresponds to volatiles (wt. %) while FC denotes fixed carbon (wt. %). The results of the proximate and ultimate analyses are presented in Table 1.
The heating value of the camel manure is compared with the heating values of a few herbivores’ manures. The reported heating values of cattle manure, goat manure, sheep manure, and horse manure are 17.01 MJ/kg, 15.27 MJ/kg, 16.02 MJ/kg, and 17.44 MJ/kg, respectively [27, 35,36,37].
The samples prior to the TGA investigation was sieved using a Haver & Boecker, Germany–made 125 micro sieve. The samples that passed through the sieve were taken for the analysis. The TG analysis was conducted under non-isothermal conditions from ambient temperature to 950 °C at various heating rates of 10, 20, and 50 °C/min. The runs were performed under an N2 atmosphere with a 100 ml/min flow rate. In all the runs, the weight of the samples was maintained between 23 and 27 mg. Each run was conducted in triplicate and the deviation among the data values was maintained at less than 5%.
2.1 Determination of lignocellulose composition of camel manure using TGA
Carrier et al. reported that the TG-DTG curves can be used to determine the lignocellulosic composition of biomass [38]. The TG-DTG curves obtained at 10 °C/min were used to determine the percentage composition of holocellulose and lignin of the camel manure sample. Based on the temperature range of zones, the weight losses (%) in each zones were calculated, and the lignocellulosic composition of the sample was estimated. The holocellulose and lignin compositions were estimated to be 55 ± 4% and 5 ± 1%, respectively. The obtained lignocellulosic compositional values are approximate values since the decomposition zones of biomass components overlap with each other during their degradation.
2.2 Theoretical backdrop—Arrhenius model
An Arrhenius model has been applied to estimate the kinetic factors of the camel manure components undergoing pyrolysis [39, 40].
The kinetics of the pyrolysis decomposition process can be represented by Eq. (2):
k can be represented by Eq. (3) as:
X can be expressed by Eq. (4):
Substituting the value of k and X in Eq. (2) yields Eq. (5):
Eq. (4) takes the form shown in Eq. (6):
The variables in Eq. (6) can be given as:
The B, C, and D are estimated employing a multiple-linear regression equation using the Linest function of Microsoft Excel [41].
3 Results and discussion
3.1 Influence of heating rate on the pyrolytic behaviour of camel manure
3.1.1 Influence of heating rate on the starting and final temperatures (decomposition) of camel manure components
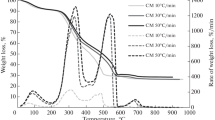
The TG-DTG curves of camel manure at 10, 20, and 50 °C/min are presented in Fig. 3.
In all the heating rates, an identical trend of TG-DTG curves can be seen. The TG curves indicate that there is a shift towards high temperature when the heating rates are increased. This shift can be ascribed to the change in the degradation temperature of substances, which behave differently for dissimilar heating rates. At low heating rates, the degradation undergoes several stages at low temperatures, while at high heating rates, the stages of decomposition occur at high temperatures. The delay in the decomposition process in high heating rates is because of the poor heat transfer. On the other hand, at low heating rates, there is an enhanced heat transfer throughout the particles and also between particles to particles [42].
It can be observed by inspection of the DTG curves that the height and width of the peaks are increasing with increasing heating rates. The high and broad shoulder peaks of high heating rates signify that the degradation rates are high at high heating rates. During small heating rates, resistance to heat and mass transfer between particles prevails. However, at high heating rates, the heat and mass transfer limitations are overcome consequently leading to improved weight loss rates. The larger shape of the peaks at high heating rates could be due to the heterogeneity and complexity of components present in the manure [43]. At high heating rates, a few components degrade simultaneously effecting an overlapping of peaks.
Figure 4 illustrates the classification of different zones of the camel manure components in the TG-DTG curves.
As illustrated in Fig. 4, the TG-DTG curves are classified into four zones constituting three endothermic shoulder peaks and one flat line. The classification of the zones was made based on the temperature range of components as each components degrade at their own temperature range. The classification was also made based on the r2 value (coefficient of correlation). The temperature ranges of zones for the various heating rates and the interpretations from Fig. 4 are well presented in Table 2.
The first zone, covering the first endotherm, corresponds to the liberation of moisture, which is confirmed by the temperature of the peak in the range 21–190 °C. The second zone comprising the next endotherm is due to the decomposition of holocellulose, which can be confirmed by its temperature range, i.e. 155–604 °C. The steep decrement of the TG curve (see Fig. 4) in zone II is attributed to the degradation of hemicellulose and cellulose. Usually in most biomass samples, the decomposition of hemicellulose occurs well below 350 °C while the decomposition of cellulose takes place between 250 and 500 °C. The third zone, constituting the last shoulder peak, indicates the degradation of lignin as it falls in the range of 565–791 °C. The fourth zone corresponds to the decomposition of char, which was formed after the devolatilisation process. A small amount of solid residue (char) remains after the fourth zone, comprising unreacted fixed carbon, and ash particles.
The observed thermal behaviour of the camel manure is compared with the thermal characteristics of other manures such as cattle, goat, and horse. Ngo et al. investigated the pyrolytic behaviour of cattle litter/manure using TGA [25]. The test was conducted from 30 to 800 °C at various heating rates (5, 10, 15, and 20 °C/min) under an N2 atmosphere (20 mL/min). Three regions were observed from the DTG curves. The first region was attributed to the dehydration of moisture, which was observed between 100 and 150 °C. The second region was noticed between 200 and 550 °C, which was ascribed to the decomposition of holocellulose and lignin. The last region was noted between 550 and 800 °C, and it was reported due to the degradation of inorganic constituents of the cattle manure. Touray et al. examined the thermochemical behaviour of goat manure by TGA [26]. The TGA tests were carried out from ambient temperature to 1000 °C at 10 °C/min under an N2 environment with 20 ml/min flow rate. Two individual stages were observed during the decomposition of the manure. The first stage was noticed between 200 and 400 °C, which was attributed to the deterioration of holocellulose. The second stage was observed between 400 and 1000 °C and was ascribed to the decomposition of lignin and low melting ashes. The pyrolytic behaviour of horse manure was examined by Chong et al. using TGA [27]. The analysis was studied at various heating rates (1, 2, 5, and 10 °C/min). All runs were conducted from ambient temperature to 900°C under an N2 medium flowing at 20 ml/min. For all the heating rates, two distinct zones were observed. The first zone was attributed to the dehydration of moisture which was noted between 30 and 110 °C. The second zone was ascribed to the devolatilisation of lignocellulosic components, which was observed between 190 and 900 °C. In the devolatilisation zone, the decomposition of holocellulose was noted between 190 and 400 °C while the degradation of lignin was noticed between 190 and 900°C. The above studies indicate that the thermochemical characteristics are different for different manures. The difference in the thermal behaviour of manures could be largely due to the difference in the composition of manures. The variation is also due to the different processing and ash pre-treatment approaches adopted by researchers.
It can be noted from the table that with increasing heating rates, the starting and ending temperatures of zones are increasing. As discussed earlier, this increase in the temperature range of zones at elevated heating rates could be attributed to the ineffective heat transfer of the particles.
3.1.2 Influence of heating rate on the pyrolysis solid product (char) yield
The quantity of the char yields after pyrolysis decomposition was 5.25 mg (22.50 %), 7.00 mg (28.28 %), and 8.64 mg (31.51 %) for 10, 20, and 50 °C/min, respectively. Thus, an increase in the char yield with increasing heating rates is observed. Oyedun et al. [44] and Mishra and Mohanty [30] also reported an increase in the char yields, when the heating rates were increased. In general, the composition of char yields increases with an increase in heating rates. Relative to higher heating rates, lower heating rates (high residence time) favour complete decomposition of the particles. At small heating rates, the slow heating leads to an effective heat transfer between intra- and inter-particles. The effective heat transfer, in turn, leads to an effective cracking/decomposition of particles subsequently leading to the release of more volatiles, thus a low char yield.
3.1.3 Influence of heating rate on combustion characteristic parameters
The characteristic factors include ignition, peak, and burnout temperatures which are critical to analysing the combustion behaviour of the samples. These parameters largely depend on the chemical composition of the material samples. Ignition temperature is the temperature at which a sample undergoes a steep fall in weight. It exhibits the ignition capability of a sample. If the ignition temperature of a sample is low, it is an indication that the sample can be ignited easily. The time corresponding to the ignition temperature is known as ‘ignition temperature time’. The temperature at which the rate of weight loss of a sample is the highest value is referred to as the ‘peak temperature’. The time period that corresponds to the peak temperature is called ‘peak temperature time’. The peak temperature and its time are important in determining the reactivity and combustibility of a sample. A low peak temperature of a sample indicates that the sample can undergo degradation easily and the degradation process is expected to be smooth and less complicated [45, 46]. The temperature at which no appreciable weight loss of a sample is observed is called the burnout temperature. The time corresponding to the burnout temperature is referred to as ‘burning time’. The burnout temperature and burning time are essential parameters as they have a significant influence on the selection of residence time and furnace temperature. The readiness of a sample to burn is indicated by the burnout temperature. A low burnout temperature means the sample can be easily burnt.
As suggested by El-Sayed and Khairy [45], the ignition, peak, and burnout temperatures of camel manure corresponding to the three heating rates are determined and their combustion characteristic parameters are presented in Table 3.
From the table, it can be noted that the ignition, peak, and burnout temperatures increase with increase in heating rates. However, the ignition temperature time, peak temperature time, and burning time decrease with increasing heating rates. The ignition, peak, and burnout temperatures shift to elevated temperatures when the heating rates are increased. This shift to higher temperatures is because of short residence periods at high heating rates. Low residence time often leads to ineffective heat transfer within and among particles. Hence, a high ignition, peak, and burnout temperatures are observed at high heating rates.
A similar thermal behaviour of samples, i.e. an increase in the ignition, peak, and burnout temperature with increasing heating rates was observed by El-Sayed and Khairy [45], Islam et al. [47], and El-Sayed et al. [48].
The reactivity, ignition, and combustion indices of camel manure corresponding to the three heating rates are estimated using the below equations (El-Sayed et al. [48].
The reactivity index is calculated by using the summation of the ratio of maximum combustion rate to peak temperature and multiplying by 100.
The ignition index is given by the ratio of maximum combustion rate to the product of time corresponding to peak temperature and ignition time.
The combustion index is given by the ratio of the product of maximum combustion rate and average combustion rate to the product of the square of ignition temperature and burnout temperature.
The calculated reactivity index, ignition index, and combustion index of camel manure for the three heating rates are presented in Table 4.
It can be observed from the table that the reactivity index decreases with increasing heating rates. In general, the sample with a high reactivity index exhibits a better reactivity [49]. Thus, it can be inferred from the study that low heating rates can induce better reactivity of samples. It can be noted that the increase in heating rate from 10 to 20 °C/min produced a slight rise in the ignition index. In general, increasing heating rates brings about a decrease in the reactivity index. Since the increase in heating rates from 10 to 20 °C/min is insignificant, only a slight increase in the ignition index was observed. However, a subsequent rise in heating rate from 20 to 50 °C/min produced a significant fall in the ignition index. This is due to the fact that more heat is released at low heating rates than at high heating rates. A similar decrease in the ignition index was also reported by El-Sayed et al. while investigating the combustion index of cotton stalk and sugarcane bagasse. Normally, the samples with a high ignition index are often easier to ignite [50]. From the study, it can be observed that at low heating rates, the samples can be ignited easily. Further, it was noticed that the rising heating rate from 10 to 20 °C/min ensued in a decrease in the combustion index. Nonetheless, a further elevation in heating rate from 20 to 50 °C/min produced an increase in the combustion index. A high combustion index means a better combustion activity. It can be inferred that a high combustion activity of samples can be achieved at high heating rates.
3.2 Influence of heating rate on conversion factor
Conversion factor can be given as:
Figure 5 illustrates the behaviour of conversion factor corresponding to heating rates 10, 20, and 50 °C/min.
An identical curve pattern was observed for all three heating rates. Until 400 °C, when the heating rate was increased, the pyrolysis reaction was slowly shifting to elevated temperatures. Nonetheless, after 400 °C, the increase in heating rate did not exhibit any influence on the behaviour of the pyrolysis reaction. Three slopes can be seen in all three curves. The first curve (< 170°C) corresponds to the release of moisture, the next curve ranging 200–530 °C corresponds to the degradation of holocellulose. The third and last curve between 530 and 775 °C corresponds to the degradation of lignin. It can be observed that the slope between 200 and 530 °C is higher than the slope between 530 and 775°C. The maximum conversion factor was achieved at 942°C, 936°C, and 923°C for 10, 20, and 50 °C/min respectively.
3.3 Influence of heating rate on the heat flow
Figure 6 illustrates the behaviour of DSC curve corresponding to the temperature rise for all the heating rates.
The DSC curves are often applied to interpret the thermal transformation that occurs during the decomposition. All the three curves followed the same behaviour by exhibiting four trends—a decreasing trend, followed by an increasing trend, subsequently followed by two decreasing trends. The first decreasing trend is due to the release of moisture, the increasing trend is because of the decomposition of holocellulose, the third (decreasing) trend is due to the degradation of lignin, and the last trend is due to the deterioration of the char. The curves indicate an active transformation during the decomposition of holocellulose, while the increasing trend of the curves shows that the decomposition of holocellulose is an exothermic reaction. The increasing trend was observed between 78 and 590 °C, 92 and 525°C, and 129 and 574°C for heating rates 10, 20, and 50 °C/min, respectively. The overall decreasing heat flow in all three curves indicates that the whole pyrolysis process is endothermic [34].
3.4 Influence of heating rate on the kinetic parameters
The calculated kinetic parameters of camel manure components for the heating rates 10, 20, and 50 °C/min are presented in Table 5.
As the vaporization of moisture is a thermodynamic phenomenon which is distinct from pyrolysis, the kinetic parameters for the dehydration process was not determined. There is no consistent trend in the behaviour of the pre-exponential factor of the manure components. For all the heating rates, the values of the pre-exponential factor of the camel manure components lie between 7.93 × 108 and 2.77 × 1013 (s-1). This demonstrates that the camel manure is composed of complex constituents, which may need different energy bands for their decomposition. Chong et al. [27] found that the pre-exponential factor values of cattle manure were in the range of 1014–1021 (s-1) and reported that the manure possesses an intricate composition.
From the table, a consistent trend in the behaviour of the activation energy of the manure components could be observed. For all the heating rates, the activation energy values were noted to be the highest (107.32–135.07 kJ/mol) for the degradation of lignin. The values of the activation energy for the degradation of holocellulose were between 38.38 and 40.74 kJ/mol. In general, a low activation energy indicates a simple and easy reaction while a high activation energy signifies a more complex and difficult reaction [51]. From the calculated activation energy values, it can be inferred that the decomposition of holocellulose is a simple process whereas the lignin decomposition is a complex one [52]. An identical observation was also reported by Yuan et al. [53]. The researchers estimated the activation energy of cattle manure employing the Kissinger-Akahira-Sunose (KAS), Friedman, Flynn-Wall-Ozawa (FWO), and Starink methods, and in all the four methods, the values of the activation energy of lignin were found to be the highest among the manure components.
The order of reaction demonstrated a consistent trend in their behaviour. The order of reaction was noted to be the highest (0.97–1.05) for the holocellulose decomposition, while it was lowest (0.45–0.56) for the lignin degradation. Wu et al. calculated the order of reaction of dairy cattle manure employing the Arrhenius model [54]. In their study too, the reaction order of holocellulose (6.37) was higher than the reaction order of lignin (2.33).
It can also be noted that there is no appreciable change in the kinetic parameter values with increasing heating rates. From the study, it can be inferred that the effect of heating rate is relatively insignificant on the kinetic parameters of camel manure pyrolysis. However, Chen et al. reported an increase in the kinetic parameter values (pre-exponential factor and activation energy) for bamboo pyrolysis, when the heating rates were increased [55]. Increasing the heating rates from 5 to 30°C/min increased the activation energy from 75.3 to 98.7 kJ/mol and the pre-exponential factor from 2.05 × 103 to 5.61 × 103 s-1. Generally, an increase in heating rates increases the kinetic parameters. According to collision theory, a rise in temperature imparts more energy to the molecules, thereby increasing the reaction rate. Damartzis et al. observed a mixed trend in the behaviour of the kinetic parameters, while studying the kinetics of cardoon pyrolysis [56]. For hemicellulose and cellulose, increasing heating rates produced an increase in the activation energy. However, for lignin increasing heating rates initially produced a decrease. A further increase in heating rates did not alter the activation energy. In the case of the pre-exponential factor, the effect of heating rate was inconsistent. El-Sayed and Khairy investigated the influence of heating rate on the kinetics of wheat dust, wheat straw, and corn cob pyrolysis [45]. It was reported that the kinetic parameter (pre-exponential factor and activation energy) behaved inconsistently with increasing heating rates. Kumar et al. studied the influence of heating rate on the kinetics of corn stover pyrolysis [57]. It was observed that the increase in heating rate brought an inconsistent trend in the values of pre-exponential factor and activation energy. However, the reaction order showed an increase with the increase in heating rates.
4 Conclusion
The present study investigated the pyrolytic behaviour of camel manure using thermogravimetric analysis under non-isothermal conditions. The influence of heating rate on the pyrolytic behaviour and kinetics on the camel manure components was also studied. The results indicated that the increasing heating rates shifted the starting and ending temperatures of manure components to elevated temperatures. The increase in heating rates shifted the ignition, peak, and burnout temperatures to higher temperatures. At the same time, the increase in heating rates improved the char yield from 22.50 to 31.51%. Increasing heating rates produced an insignificant effect on the kinetic parameters of the camel manure components.
The pyrolysis characteristics of camel manure provide fundamental knowledge on the decomposition mechanism and reaction parameters. The information can be used to design and fabricate pyrolysers and optimise the pyrolysis process operating conditions and in simulation studies to calculate the heat and mass balances of the camel manure pyrolysis. Future research should focus on studying the effect of particle size, and other inert atmospheres such as helium on the pyrolytic behaviour of camel manure. Upcoming studies should employ using other models such as Friedman, Kissinger-Akahira Sunnose, Discrete Activation energy, and Flynn-Wall-Ozawa, to determine the kinetic parameters. It can be inferred from the study that the pyrolysis of camel manure yields a substantial quantity of char; however, the properties of camel manure char need to be investigated for their suitability as a soil conditioner.
Data availability
The TGA data file has been submitted as a separate file.
Code availability
Not applicable.
References
(2018) Agriculture Statistics, Planning and Statistics Authority, Qatar. Doha
Lensch J (1999) The two-humped camel (Camelus bactrianus). World Anim Rev 92:36–41
Parthasarathy P, Fernandez A, Al-Ansari T et al (2021) Thermal degradation characteristics and gasification kinetics of camel manure using thermogravimetric analysis. J Environ Manag 287:112345. https://doi.org/10.1016/J.JENVMAN.2021.112345
Abdel-Rahman EH, Alzahrani FS, Sulieman AME (2020) Potential of camel dung as promising organic manure in Saudi Arabia. Adv Life Sci 7:202–207
Kakar AR (2016) Camels’ manure-from waste to a worthwhile farming agent. https://arkbiodiv.com/2016/02/02/camels-dungzfrom-waste-to-a-worthwhile-farmingagent/#:~:text=Camel%20dung%20is%20beautiful%20in,especially%20among%20the%20pastoralists'%20communities.&text=Camels'%20dung%20is%20used%20for,but%20at%20a%20minor%20leve. Accessed 9 Sep 2020
Safieddin Ardebili SM, Khademalrasoul A (2020) An assessment of feasibility and potential of gaseous biofuel production from agricultural/animal wastes: a case study. Biomass Convers Biorefinery:1–10. https://doi.org/10.1007/s13399-020-00901-z
Parthasarathy P, Narayanan KS (2014) Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield - a review. Renew Energy 66:570–579. https://doi.org/10.1016/j.renene.2013.12.025
Parthasarathy P, Sheeba KN (2015) Combined slow pyrolysis and steam gasification of biomass for hydrogen generation-a review. Int J Energy Res 39. https://doi.org/10.1002/er.3218
Elkhalifa S, Al-Ansari T, Mackey HR, Mckay G (2019) Food waste to biochars through pyrolysis : a review. Resour Conserv Recycl 144:310–320
Shahbaz M, AlNouss A, Parthasarathy P, Abdelaal AH, Mackey H, McKay G, al-Ansari T (2020) Investigation of biomass components on the slow pyrolysis products yield using Aspen Plus for techno-economic analysis. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01040-1
Elkhalifa S, AlNouss A, Al-Ansari T, Mackey HR, Parthasarathy P, Mckay G (2019) Simulation of food waste pyrolysis for the production of biochar: A Qatar case study. Comput Aided Chem Eng 46:901–906
Şen AU, Nobre C, Durão L, Miranda I, Pereira H, Gonçalves M (2020) Low-temperature biochars from cork-rich and phloem-rich wastes: fuel, leaching, and methylene blue adsorption properties. Biomass Convers Biorefinery:1–11. https://doi.org/10.1007/s13399-020-00949-x
AlNouss A, Parthasarathy P, Mackey HR, al-Ansari T, McKay G (2021) Pyrolysis study of different fruit wastes using an Aspen Plus Model. Front Sustain Food Syst 5:604001. https://doi.org/10.3389/fsufs.2021.604001
Waqas M, Asam Z, Rehan M et al (2020) Development of biomass-derived biochar for agronomic and environmental remediation applications. Biomass Convers Biorefinery:1–23. https://doi.org/10.1007/s13399-020-00936-2
Pighinelli ALMT, Boateng AA, Mullen CA, Elkasabi Y (2014) Evaluation of Brazilian biomasses as feedstocks for fuel production via fast pyrolysis. Energy Sustain Dev 21:42–50. https://doi.org/10.1016/j.esd.2014.05.002
Fernandez A, Saffe A, Pereyra R, Mazza G, Rodriguez R (2016) Kinetic study of regional agro-industrial wastes pyrolysis using non-isothermal TGA analysis. Appl Therm Eng 106:1157–1164. https://doi.org/10.1016/j.applthermaleng.2016.06.084
Chaudhary RG, Juneja HD, Pagadala R, Gandhare NV, Gharpure MP (2014) Synthesis, characterisation and thermal degradation behaviour of some coordination polymers by using TG-DTG and DTA techniques. J Saudi Chem Soc 19:442–453. https://doi.org/10.1016/j.jscs.2014.06.002
Ferreira RA d R, Meireles C d S, Assunção RMN et al (2020) Optimization of the oxidative fast pyrolysis process of sugarcane straw by TGA and DSC analyses. Biomass Bioenergy 134:105456. https://doi.org/10.1016/j.biombioe.2019.105456
Sahoo A, Kumar S, Mohanty K (2020) A comprehensive characterization of non-edible lignocellulosic biomass to elucidate their biofuel production potential. Biomass Convers Biorefinery:1–17. https://doi.org/10.1007/s13399-020-00924-6
Grioui N, Halouani K, Agblevor FA (2014) Bio-oil from pyrolysis of Tunisian almond shell: comparative study and investigation of aging effect during long storage. Energy Sustain Dev 21:100–112. https://doi.org/10.1016/j.esd.2014.05.006
Ulusal A, Apaydın Varol E, Bruckman VJ, Uzun BB (2020) Opportunity for sustainable biomass valorization to produce biochar for improving soil characteristics. Biomass Convers Biorefinery:1–11. https://doi.org/10.1007/s13399-020-00923-7
Hong-bo L, Guang-yu Z, Chun-xia J (2009) Analysis on TG-FTIR and kinetics of biomass pyrolysis Publisher. In: 2009 International Conference on Sustainable Power Generation and Supply. IEEE, Nanjing, China, pp 1–5
Wang L, Shahbazi A, Hanna MA (2011) Characterization of corn stover, distiller grains and cattle manure for thermochemical conversion. Biomass Bioenergy 35:171–178. https://doi.org/10.1016/j.biombioe.2010.08.018
Jeguirim M, Bikai J, Elmay Y, Limousy L, Njeugna E (2014) Thermal characterization and pyrolysis kinetics of tropical biomass feedstocks for energy recovery. Energy Sustain Dev 23:188–193. https://doi.org/10.1016/j.esd.2014.09.009
Ngo T, Kim S, Kim S (2010) Characteristics and kinetics of cattle litter pyrolysis in a tubing reactor. Bioresour Technol 101:S104–S108
Touray N, Tsai WT, Li MH (2014) Effect of holding time during pyrolysis on thermochemical and physical properties of biochars derived from goat manure. Waste Biomass Valorization 5:1029–1033. https://doi.org/10.1007/s12649-014-9315-6
Chong CT, Mong GR, Ng JH, Chong WWF, Ani FN, Lam SS, Ong HC (2019) Pyrolysis characteristics and kinetic studies of horse manure using thermogravimetric analysis. Energy Convers Manag 180:1260–1267. https://doi.org/10.1016/j.enconman.2018.11.071
Tu D, Dong H, Shang B (2008) Pyrolysis behavior of selected manures using TG-FTIR techniques. In: Livestock Environment VIII Conference. 31 August - 4 September 2008, Iguassu Falls, Brazil
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19
Mishra RK, Mohanty K (2018) Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour Technol 251:63–74. https://doi.org/10.1016/j.biortech.2017.12.029
Fernandez A, Ortiz LR, Asensio D, Rodriguez R, Mazza G (2020) Kinetic analysis and thermodynamics properties of air/steam gasification of agricultural waste. J Environ Chem Eng 8:103829. https://doi.org/10.1016/j.jece.2020.103829
Jahirul MI, Rasul MG, Chowdhury AA, Ashwath N (2012) Biofuels production through biomass pyrolysis —A technological review. Energies 5:4952–5001
Eke J, Onwudili JA, Bridgwater AV (2020) Influence of moisture contents on the fast pyrolysis of trommel fines in a bubbling fluidized bed reactor. Waste Biomass Valorization 11:3711–3722
Ahmad MS, Mehmood MA, Luo H, Shen B, Latif M, Ghani WAWAK, Alkhattabi NA, Aloqbi AA, Jambi EJ, Gull M, Rashid U (2019) Pyrolysis and thermogravimetric study to elucidate the bioenergy potential of novel feedstock produced on poor soils while keeping the environmental sustainability intact. Sustainability 11(13):3592
Chen G, He S, Cheng Z, Guan Y, Yan B, Ma W, Leung DYC (2017) Comparison of kinetic analysis methods in thermal decomposition of cattle manure by themogravimetric analysis. Bioresour Technol 243:69–77. https://doi.org/10.1016/j.biortech.2017.06.007
Tu D, Dong H, Wid BS (2008) Pyrolysis behavior of selected manures using TG-FTTR techniques. In: Livestock Environment VIII - Proceedings of the 8th International Symposium. pp 215–222
(2009) Phyllis2 - sheep manure (#2772). In: ECN (Energy Res. Cent. Netherlands). https://phyllis.nl/Biomass/View/2834. Accessed 18 Apr 2019
Carrier M, Loppinet-Serani A, Denux D, Lasnier JM, Ham-Pichavant F, Cansell F, Aymonier C (2011) Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 35:298–307. https://doi.org/10.1016/j.biombioe.2010.08.067
Parthasarathy P, Choi HS, Hwang JG, Park HC (2017) Determination of thermal decomposition kinetics of low grade coal employing thermogravimetric analysis. Korean J Chem Eng 34:1678–1692
Parthasarathy P, Narayanan KS, Arockiam L (2013) Study on kinetic parameters of different biomass samples using thermo-gravimetric analysis. Biomass Bioenergy 58:58–66. https://doi.org/10.1016/j.biombioe.2013.08.004
Goenka R, Parthasarathy P, Gupta NK, Biyahut NK, Narayanan S (2015) Kinetic analysis of biomass and comparison of its chemical compositions by thermogravimetry, wet and experimental furnace methods. Waste Biomass Valorization 6:989–1002. https://doi.org/10.1007/s12649-015-9402-3
Lin L, Gustafsson E, Strand M (2011) Aerosol-based method for investigating biomass char reactivity at high temperatures. Combust Flame 158:1426–1437
Liang Y, Cheng B, Si Y, Cao DJ, Jiang HY, Han GM, Liu XH (2014) Thermal decomposition kinetics and characteristics of Spartina alterni fl ora via thermogravimetric analysis. Renew Energy 68:111–117. https://doi.org/10.1016/j.renene.2014.01.041
Oyedun AO, Gebreegziabher T, Hui D (2013) Mechanism and modelling of bamboo pyrolysis. Fuel Process Technol 106:595–604
El-sayed SA, Khairy M (2015) Effect of heating rate on the chemical kinetics of different biomass pyrolysis materials. Biofuels 6:157–170. https://doi.org/10.1080/17597269.2015.1065590
El-Sayed SA, Ismail MA, Mostafa ME (2019) Thermal decomposition and combustion characteristics of biomass materials using TG/DTG at different high heating rates and sizes in the air. Environ Prog Sustain Energy 38:13124. https://doi.org/10.1002/ep.13124
Islam A, Auta M, Kabir G, Hameed BH (2016) Bioresource Technology A thermogravimetric analysis of the combustion kinetics of karanja ( Pongamia pinnata ) fruit hulls char. Bioresour Technol 200:335–341. https://doi.org/10.1016/j.biortech.2015.09.057
El-sayed SA, Ismail MA, Mostafa ME (2018) Thermal decomposition and combustion characteristics of biomass materials using TG / DTG at Different High Heating Rates and Sizes in the Air. Environ Prog Sustain 38:1–14. https://doi.org/10.1002/ep.13124
Wang Q, Zhao W, Liu H, Jia C, Xu H (2012) Reactivity and kinetic analysis of biomass during combustion. Energy Procedia 17:869–875
Protásio T d P, Junior MG, Mirmehdi S et al (2017) Combustion of biomass and charcoal made from Babassu nutshell. CERNE 23:1–10
Zhang X, Xu M, Sun R, Sun L (2006) Study on biomass pyrolysis kinetics. J Eng Gas Turbines Power 128(128):493–496
Wang L, Zhang R, Li J, Guo L, Yang H, Ma F, Yu H (2018) Comparative study of the fast pyrolysis behavior of ginkgo, poplar, and wheat straw lignin at different temperatures. Ind Crop Prod 122:465–472. https://doi.org/10.1016/j.indcrop.2018.06.038
Yuan X, He T, Cao H, Yuan Q (2017) Cattle manure pyrolysis process: kinetic and thermodynamic analysis with isoconversional methods. Renew Energy 107:489–496. https://doi.org/10.1016/j.renene.2017.02.026
Wu H, Hanna MA, Jones DD (2012) Thermogravimetric characterization of dairy manure as pyrolysis and combustion feedstocks. Waste Manag Res 30:1066–1071
Chen D, Zhou J, Zhang Q (2014) Bioresource Technology Effects of heating rate on slow pyrolysis behavior , kinetic parameters and products properties of moso bamboo. Bioresour Technol 169:313–319. https://doi.org/10.1016/j.biortech.2014.07.009
Damartzis T, Vamvuka D, Sfakiotakis S, Zabaniotou A (2011) Thermal degradation studies and kinetic modeling of cardoon ( C ynara cardunculus ) pyrolysis using thermogravimetric analysis ( TGA ). Bioresour Technol 102:6230–6238. https://doi.org/10.1016/j.biortech.2011.02.060
Kumar A, Wang L, Dzenis YA et al (2008) Thermogravimetric characterization of corn stover as gasification and pyrolysis feedstock. Biomass Bioenergy 32:460–467
Acknowledgements
The authors are grateful to Hamad Bin Khalifa University (HBKU) and Qatar Foundation (QF) for their support for the research work. The authors appreciate Hamad Bin Khalifa University (HBKU), Qatar Foundation (QF), and the Supreme Committee for Delivery and Legacy (SCDL) for their financial support. The authors would like to thank Qatar National Research Fund for their support of this research through NPRP-11S-0117-180328.
Funding
Open access funding provided by the Qatar National Library.
Author information
Authors and Affiliations
Contributions
Prakash Parthasarathy: lead data collection, lead data interpretation, and lead manuscript preparation.
Hamish R. Mackey: project concept, project design, and manuscript revision.
Tareq Al-Ansari: project concept, project design, and manuscript revision.
Gordon McKay: project lead, project concept, project design, data interpretation, and manuscript revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parthasarathy, P., Al-Ansari, T., Mackey, H.R. et al. Effect of heating rate on the pyrolysis of camel manure. Biomass Conv. Bioref. 13, 6023–6035 (2023). https://doi.org/10.1007/s13399-021-01531-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01531-9