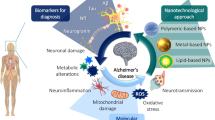
Abstract
Alzheimer's disease (AD) is one of the neurodegenerative diseases that leads to the deposition of amyloid plaques in the neuropil as well as outside the cells in the brain leading to sometimes cell death. Even though there are various traditional diagnosis methods, they lack sensitivity towards the detection of AD. FDA has also approved some drugs for the treatment and alleviation of AD severity but they are not capable of curing AD. The use of nanotechnology for biomedical applications such as specific drug delivery vehicles to treat various neurodegenerative disorders including Alzheimer's disease (AD) and also nanotechnology offers highly sensitive nanodiagnostic tools that utilize different nanoparticles/nanostructures. AD therapy utilizing a multifunctional nanotechnology approach could be developed for designing therapeutic cocktails and diagnostic tools (nanosensors, imaging) that simultaneously and specifically target the important molecule involved in AD. The interaction of different functionalized nanostructures used for the therapy of AD and its different physicochemical interactions with the neuronal cells in vitro or in vivo needs to be discussed. In this review, we will briefly discuss about the genes/proteins that are involved in AD and its pathology, traditional AD diagnosis methods such as magnetic resonance imaging, PET scan, in vitro nanodiagnostic methods such as zinc oxide nanoflower platform, surface plasmon resonance nanoparticle, scanning tunnelling microscopy, and QCM-based detection. In vivo nanodiagnostic approaches such as the use of nanoparticles in AD diagnosis with MRI, optical imaging, quantum dots, nanotechnology-based drug delivery systems for the treatment of AD such as solid lipid nanoparticles, liposomes, polymeric nanoparticles, nanogels, fullerenes, nano-ceria, dendrimers, zinc oxide nanoflowers, gold nanoparticles, FDA-approved drugs and nasal route of drug administration for effective AD treatment will also be discussed.




Similar content being viewed by others
References
Aderibigbe, B. A., & Naki, T. (2018). Design and efficacy of nanogels formulations for intranasal administration. Molecules, 23(6), 1241.
Akhtar, N., Metkar, S. K., Girigoswami, A., & Girigoswami, K. (2017). ZnO nanoflower based sensitive nano-biosensor for amyloid detection. Materials Science and Engineering C, 78, 960–968. https://doi.org/10.1016/j.msec.2017.04.118
Akkus, Z., Galimzianova, A., Hoogi, A., Rubin, D. L., & Erickson, B. J. (2017). Deep learning for brain MRI segmentation: State of the art and future directions. Journal of digital imaging, 30(4), 449–459.
Aliev, G., Ashraf, G. M., Tarasov, V. V., Chubarev, V. N., Leszek, J., Gasiorowski, K., Makhmutova, A., Baeesa, S. S., Avila-Rodriguez, M., & Ustyugov, A. A. (2019). Alzheimer’s Disease-Future Therapy Based on Dendrimers. Current Neuropharmacology, 17(3), 288–294.
Ansari, M. A., & Scheff, S. W. (2010). Oxidative stress in the progression of Alzheimer disease in the frontal cortex. Journal of Neuropathology & Experimental Neurology, 69(2), 155–167.
Appel, J., Potter, E., Shen, Q., Pantol, G., Greig, M. T., Loewenstein, D., & Duara, R. (2009). A comparative analysis of structural brain MRI in the diagnosis of Alzheimer’s disease. Behavioural neurology, 21(1, 2), 13–19.
Brambilla, D., le Droumaguet, B., Nicolas, J., Hashemi, S. H., Wu, L.-P., Moghimi, S. M., Couvreur, P., & Andrieux, K. (2011). Nanotechnologies for Alzheimer’s disease: diagnosis, therapy, and safety issues. Nanomedicine Nanotechnology Biology and Medicine, 7(5), 521–540.
Canevelli, M., Piscopo, P., Talarico, G., Vanacore, N., Blasimme, A., Crestini, A., Tosto, G., Troili, F., Lenzi, G. L., & Confaloni, A. (2014). Familial Alzheimer’s disease sustained by presenilin 2 mutations: Systematic review of literature and genotype–phenotype correlation. Neuroscience & Biobehavioral Reviews, 42, 170–179.
Charbgoo, F., Bin, A. M., & Darroudi, M. (2017). Cerium oxide nanoparticles: green synthesis and biological applications. International journal of nanomedicine, 12, 1401.
de Leon, M. J., DeSanti, S., Zinkowski, R., Mehta, P. D., Pratico, D., Segal, S., Clark, C., Kerkman, D., DeBernardis, J., & Li, J. (2004). MRI and CSF studies in the early diagnosis of Alzheimer’s disease. Journal of internal medicine, 256(3), 205–223.
de Reuck, J. L., Deramecourt, V., Auger, F., Durieux, N., Cordonnier, C., Devos, D., Defebvre, L., Moreau, C., Caparros-Lefebvre, D., & Leys, D. (2014). Iron deposits in post-mortem brains of patients with neurodegenerative and cerebrovascular diseases: a semi-quantitative 7.0 T magnetic resonance imaging study. European journal of neurology, 21(7), 1026–1031.
den Haan, J., Morrema, T. H. J., Verbraak, F. D., de Boer, J. F., Scheltens, P., Rozemuller, A. J., Bergen, A. A. B., Bouwman, F. H., & Hoozemans, J. J. (2018). Amyloid-beta and phosphorylated tau in post-mortem Alzheimer’s disease retinas. Acta neuropathologica communications, 6(1), 1–11.
dos Santos, T. N., da Silva, S., Arruda, R., Ugioni, K. S., Canteiro, P. B., de Bem, S. G., Mendes, C., Silveira, P. C. L., & Muller, A. P. (2020). Gold nanoparticles treatment reverses brain damage in Alzheimer’s disease model. Molecular neurobiology, 57(2), 926–936.
Dowding, J. M., Song, W., Bossy, K., Karakoti, A., Kumar, A., Kim, A., Bossy, B., Seal, S., Ellisman, M. H., & Perkins, G. (2014). Cerium oxide nanoparticles protect against A β-induced mitochondrial fragmentation and neuronal cell death. Cell Death & Differentiation, 21(10), 1622–1632.
Dykman LA, Khlebtsov NG (2011) Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae (aнглoязычнaя вepcия) 3(2 (9))
Efthymiou, A. G., & Goate, A. M. (2017). Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Molecular neurodegeneration, 12(1), 1–12.
Erdő, F., Bors, L. A., Farkas, D., Bajza, Á., & Gizurarson, S. (2018). Evaluation of intranasal delivery route of drug administration for brain targeting. Brain research bulletin, 143, 155–170.
Escamilla-Ayala, A. A., Sannerud, R., Mondin, M., Poersch, K., Vermeire, W., Paparelli, L., Berlage, C., Koenig, M., Chavez-Gutierrez, L., & Ulbrich, M. H. (2020). Super-resolution microscopy reveals majorly mono-and dimeric presenilin1/γ-secretase at the cell surface. eLife, 9, e56679–e56679.
Farkhondeh T, Forouzanfar F, Roshanravan B, Samarghandian S (2019) Curcumin effect on non-amyloidogenic pathway for preventing alzheimer’s disease. Biointerface Research in Applied Chemistry 9(4):4085–4089 http://www.funrich.org
Fonseca, L. C., Lopes, J. A., Vieira, J., Viegas, C., Oliveira, C. S., Hartmann, R. P., & Fonte, P. (2021). Intranasal drug delivery for treatment of Alzheimer’s disease. Drug Delivery and Translational Research, 11(2), 411–425.
Forner, S., Baglietto-Vargas, D., Martini, A. C., Trujillo-Estrada, L., & LaFerla, F. M. (2017). Synaptic impairment in Alzheimer’s disease: A dysregulated symphony. Trends in neurosciences, 40(6), 347–357.
Giorgio, A., & de Stefano, N. (2013). Clinical use of brain volumetry. Journal of Magnetic Resonance Imaging, 37(1), 1–14.
Girigoswami, A., Ramalakshmi, M., Akhtar, N., Metkar, S. K., & Girigoswami, K. (2019). ZnO Nanoflower petals mediated amyloid degradation - an in vitro electrokinetic potential approach. Materials Science and Engineering C, 101, 169–178. https://doi.org/10.1016/j.msec.2019.03.086
Girigoswami, K., Ku, S. K., Ryu, J., & Park, C. B. (2008). A synthetic amyloid lawn system for high-throughput analysis of amyloid toxicity and drug screening. Biomaterials, 29, 2813–2819. https://doi.org/10.1016/j.biomaterials.2008.03.022
Gleerup, HS, Hasselbalch, SG, Simonsen, AH. (2019). Biomarkers for Alzheimer’s disease in saliva: A systematic review. Disease Markers. https://doi.org/10.1155/2019/4761054
Güner G, Lichtenthaler SF (2020) The substrate repertoire of γ-secretase/presenilin. In: Seminars in Cell & Developmental Biology. Elsevier
Guo, X., Lie, Q., Liu, Y., Jia, Z., Gong, Y., Yuan, X., & Liu, J. (2021). Multifunctional selenium quantum dots for the treatment of Alzheimer’s disease by reducing Aβ-neurotoxicity and oxidative stress and alleviate neuroinflammation. ACS Applied Materials & Interfaces, 13(26), 30261–30273.
Hadavi, D., & Poot, A. A. (2016). Biomaterials for the Treatment of Alzheimer’s Disease. Frontiers in bioengineering and biotechnology, 4, 49.
Haes, A. J., Chang, L., Klein, W. L., & van Duyne, R. P. (2005). Detection of a biomarker for Alzheimer’s disease from synthetic and clinical samples using a nanoscale optical biosensor. Journal of the American Chemical Society, 127(7), 2264–2271.
Hajipour, M. J., Santoso, M. R., Rezaee, F., Aghaverdi, H., Mahmoudi, M., & Perry, G. (2017). Advances in alzheimer’s diagnosis and therapy: The implications of nanotechnology. Trends in biotechnology, 35(10), 937–953.
Haribabu, V., Girigoswami, K., Sharmiladevi, P., & Girigoswami, A. (2020). Water-Nanomaterial Interaction to Escalate Twin-Mode Magnetic Resonance Imaging. ACS Biomaterials Science & Engineering, 6(8), 4377–4389.
Harilal, S., Jose, J., Parambi, D. G. T., Kumar, R., Mathew, G. E., Uddin, M. S., Kim, H., & Mathew, B. (2019). Advancements in nanotherapeutics for Alzheimer’s disease: Current perspectives. Journal of Pharmacy and Pharmacology, 71(9), 1370–1383.
Herholz, K., Salmon, E., Perani, D., Baron, J.-C., Holthoff, V., Frölich, L., Schönknecht, P., Ito, K., Mielke, R., & Kalbe, E. (2002). Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. NeuroImage, 17(1), 302–316.
Hernandez-Sapiens, M. A., Reza-Zaldívar, E. E., Márquez-Aguirre, A. L., Gómez-Pinedo, U., Matias-Guiu, J., Cevallos, R. R., Mateos-Díaz, J. C., Sánchez-González, V. J., & Canales-Aguirre, A. A. (2022). Presenilin mutations and their impact on neuronal differentiation in Alzheimer’s disease. Neural Regeneration Research, 17(1), 31.
Hu, B., Dai, F., Fan, Z., Ma, G., Tang, Q., & Zhang, X. (2015). Nanotheranostics: Congo red/Rutin-MNPs with enhanced magnetic resonance imaging and H2O2-responsive therapy of Alzheimer’s disease in APPswe/PS1dE9 transgenic mice. Advanced Materials, 27(37), 5499–5505.
Huy, P. D. Q., & Li, M. S. (2014). Binding of fullerenes to amyloid beta fibrils: Size matters. Physical Chemistry Chemical Physics, 16(37), 20030–20040.
Hwang, S. S., Chan, H., Sorci, M., van Deventer, J., Wittrup, D., Belfort, G., & Walt, D. (2019). Detection of amyloid β oligomers toward early diagnosis of Alzheimer’s disease. Analytical biochemistry, 566, 40–45.
Islam J, Zhang Y (2019) Understanding 3D CNN behavior for Alzheimer’s disease diagnosis from brain PET scan. arXiv preprint arXiv:191204563
Israel, L. L., Galstyan, A., Holler, E., & Ljubimova, J. Y. (2020). Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. Journal of Controlled Release, 320, 45–62.
Jaiswal, J. K., & Simon, S. M. (2004). Potentials and pitfalls of fluorescent quantum dots for biological imaging. Trends in cell biology, 14(9), 497–504.
Jouanne, M., Rault, S., & Voisin-Chiret, A.-S. (2017). Tau protein aggregation in Alzheimer’s disease: An attractive target for the development of novel therapeutic agents. European journal of medicinal chemistry, 139, 153–167.
Kabanov, A. V., & Vinogradov, S. V. (2009). Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angewandte Chemie International Edition, 48(30), 5418–5429.
Kamaly, N., Xiao, Z., Valencia, P. M., Radovic-Moreno, A. F., & Farokhzad, O. C. (2012). Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chemical Society Reviews, 41(7), 2971–3010.
Kang, H., Park, T., Choi, I., Lee, Y., Ito, E., Hara, M., & Noh, J. (2009). Formation of large ordered domains in benzenethiol self-assembled monolayers on Au (1 1 1) observed by scanning tunneling microscopy. Ultramicroscopy, 109(8), 1011–1014.
Kim, Y. H., Lee, S.-M., Cho, S., Kang, J.-H., Minn, Y.-K., Park, H., & Choi, S. H. (2019). Amyloid beta in nasal secretions may be a potential biomarker of Alzheimer’s disease. Scientific reports, 9(1), 1–9.
Klajnert, B., Cortijo-Arellano, M., Cladera, J., & Bryszewska, M. (2006). Influence of dendrimer’s structure on its activity against amyloid fibril formation. Biochemical and biophysical research communications, 345(1), 21–28.
Korolev, I. O. (2014). Alzheimer’s disease: A clinical and basic science review. Medical Student Research Journal, 4(1), 24–33.
Kotormán, M., Romhányi, D., Alpek, B., et al. (2021). Fruit juices are effective anti-amyloidogenic agents. Biologia Futura, 72, 257–262. https://doi.org/10.1007/s42977-020-00064-y
Laske, C., Sohrabi, H. R., Frost, S. M., López-de-Ipiña, K., Garrard, P., Buscema, M., Dauwels, J., Soekadar, S. R., Mueller, S., & Linnemann, C. (2015). Innovative diagnostic tools for early detection of Alzheimer’s disease. Alzheimer’s & Dementia, 11(5), 561–578.
Lee, J. C., Kim, S. J., Hong, S., & Kim, Y. (2019). Diagnosis of Alzheimer’s disease utilizing amyloid and tau as fluid biomarkers. Experimental & molecular medicine, 51(5), 1–10.
Lee, J.-H., Kang, D.-Y., Kim, S.-U., Yea, C.-H., Oh, B.-K., & Choi, J.-W. (2009). Electrical detection of β-amyloid (1–40) using scanning tunneling microscopy. Ultramicroscopy, 109(8), 923–928.
Liebman JF, Severin K, Klapötke TM (2003) Inorganic Exotic Molecules. In: Encyclopedia of Physical Science and Technology. Elsevier, pp 817–838
Loureiro, J. A., Andrade, S., Duarte, A., Neves, A. R., Queiroz, J. F., Nunes, C., Sevin, E., Fenart, L., Gosselet, F., & Coelho, M. A. N. (2017). Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules, 22(2), 277.
Metkar, S. K., Girigoswami, A., Murugesan, R., & Girigoswami, K. (2017a). In vitro and in vivo insulin amyloid degradation mediated by Serratiopeptidase. Materials Science and Engineering C, 70, 728–735. https://doi.org/10.1016/j.msec.2016.09.049
Metkar SK, Girigoswami A, Murugesan R, Girigoswami K (2017b) Lumbrokinase for degradation and reduction of amyloid fibrils associated with amyloidosis.15:96–104. https://doi.org/10.1016/j.jab.2017.01.003
Metkar, S. K., Ghosh, S., Girigoswami, A., & Girigoswami, K. (2019). Prion Peptide 106–126 Degradation Potential of Serratiopetidase and Lumbrokinase - an In Vitro and In Silico Perspective. CNS & Neurological Disorders - Drug Targets, 18(9), 723–731. https://doi.org/10.2174/1871527318666191021150002
Metkar, S. K., Girigoswami, A., Vijayashree, R., & Girigoswami, K. (2020). Attenuation of subcutaneous insulin induced amyloid mass in vivo using Lumbrokinase and Serratiopeptidase. Int J of Biol Macromolecules, 163, 128–134. https://doi.org/10.1016/j.ijbiomac.2020.06.256
Madaan, K., Kumar, S., Poonia, N., Lather, V., & Pandita, D. (2014). Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. Journal of pharmacy & bioallied sciences, 6(3), 139.
Michalet, X., Pinaud, F. F., Bentolila, L. A., Tsay, J. M., Doose, S., Li, J. J., Sundaresan, G., Wu, A. M., Gambhir, S. S., & Weiss, S. (2005). Quantum dots for live cells, in vivo imaging, and diagnostics. Science, 307(5709), 538–544.
Mourtas, S., Canovi, M., Zona, C., Aurilia, D., Niarakis, A., la Ferla, B., Salmona, M., Nicotra, F., Gobbi, M., & Antimisiaris, S. G. (2011). Curcumin-decorated nanoliposomes with very high affinity for amyloid-β1-42 peptide. Biomaterials, 32(6), 1635–1645.
Nazem, A., & Mansoori, G. A. (2008). Nanotechnology solutions for Alzheimer’s disease: Advances in research tools, diagnostic methods and therapeutic agents. Journal of Alzheimer’s disease, 13(2), 199–223.
Nazem, A., & Mansoori, G. A. (2011). Nanotechnology for Alzheimer’s disease detection and treatment. Insciences J, 1(4), 169–193.
Neha, B., Ganesh, B., & Preeti, K. (2013). Drug delivery to the brain using polymeric nanoparticles: A review. International Journal of Pharmaceutical and Life Sciences, 2(3), 107–132.
Nesterov, E. E., Skoch, J., Hyman, B. T., Klunk, W. E., Bacskai, B. J., & Swager, T. M. (2005). In vivo optical imaging of amyloid aggregates in brain: Design of fluorescent markers. Angewandte Chemie International Edition, 44(34), 5452–5456.
Park CB, Ku SH, Girigoswami K, Ryu J (2011) Method for screening drug for neurodegenerative diseases treatment. Korean Patent: Appl. No. 10–2007–0112804 (2007. 11. 06) Patent No. 10–1082484–0000 (2011. 11. 02)
Nordberg, A., Rinne, J. O., Kadir, A., & Långström, B. (2010). The use of PET in Alzheimer disease. Nature Reviews Neurology, 6(2), 78–87.
Patel, D. A., Henry, J. E., & Good, T. A. (2007). Attenuation of β-amyloid-induced toxicity by sialic-acid-conjugated dendrimers: Role of sialic acid attachment. Brain research, 1161, 95–105.
Pathak M, Singhal R (2022). Therapeutic and diagnostic applications of nanocomposites in the treatment Alzheimer’s disease studies. Biointerface Research in Applied Chemistry 12(1), 940–960.
Pet Imaging In Alzheimer’s Disease (2013) https://www.bangkokmedjournal.com/article/pet-imaging-in-alzheimer-rsquo-s-disease/152/article. Vol 5. Accessed on 25.05.2022.
Picone, P., Ditta, L. A., Sabatino, M. A., Militello, V., San Biagio, P. L., di Giacinto, M. L., Cristaldi, L., Nuzzo, D., Dispenza, C., & Giacomazza, D. (2016). Ionizing radiation-engineered nanogels as insulin nanocarriers for the development of a new strategy for the treatment of Alzheimer’s disease. Biomaterials, 80, 179–194.
Picone, P., Sabatino, M. A., Ditta, L. A., Amato, A., San Biagio, P. L., Mulè, F., Giacomazza, D., Dispenza, C., & di Carlo, M. (2018). Nose-to-brain delivery of insulin enhanced by a nanogel carrier. Journal of Controlled Release, 270, 23–36.
Piñero, J., Ramírez-Anguita, J. M., Saüch-Pitarch, J., Ronzano, F., Centeno, E., Sanz, F., & Furlong, L. I. (2020). The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic acids research, 48(D1), D845–D855.
Pleskova S, Mikheeva E, Gornostaeva E (2018) Using of quantum dots in biology and medicine. Cellular and Molecular Toxicology of Nanoparticles 323–34.
Podolski, I. Y., Podlubnaya, Z. A., Kosenko, E. A., Mugantseva, E. A., Makarova, E. G., Marsagishvili, L. G., Shpagina, M. D., Kaminsky, Y. G., Andrievsky, G. V., & Klochkov, V. K. (2007). Effects of hydrated forms of C60 fullerene on amyloid β-peptide fibrillization in vitro and performance of the cognitive task. Journal of nanoscience and nanotechnology, 7(4–5), 1479–1485.
Rabiee, N., Ahmadi, S., Afshari, R., Khalaji, S., Rabiee, M., Bagherzadeh, M., Fatahi, Y., Dinarvand, R., Tahriri, M., & Tayebi, L. (2021). Polymeric Nanoparticles for Nasal Drug Delivery to the Brain: Relevance to Alzheimer’s Disease. Advanced Therapeutics, 4(3), 2000076.
Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. (2021). Population estimate of people with clinical Alzheimer's disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s & Dementia 17(12), 1966–1975. https://doi.org/10.1002/alz.12362
Rotman, M., Snoeks, T. J. A., & van der Weerd, L. (2011). Pre-clinical optical imaging and MRI for drug development in Alzheimer’s disease. Drug Discovery Today: Technologies, 8(2–4), e117–e125.
Rutegård, M. K., Båtsman, M., Axelsson, J., Brynolfsson, P., Brännström, F., Rutegård, J., Ljuslinder, I., Blomqvist, L., Palmqvist, R., & Rutegård, M. (2019). PET/MRI and PET/CT hybrid imaging of rectal cancer–description and initial observations from the RECTOPET (REctal Cancer trial on PET/MRI/CT) study. Cancer Imaging, 19(1), 1–9.
Safenkova, I. V., Zherdev, A. V., & Dzantiev, B. B. (2019). Using atomic force microscopy to assess surface modification of gold nanoparticles. Biointerface Res App Chem, 9, 3894–3897.
Saini, S., Sharma, T., Jain, A., Kaur, H., Katare, O. P., & Singh, B. (2021). Systematically designed chitosan-coated solid lipid nanoparticles of ferulic acid for effective management of Alzheimer’s disease: A preclinical evidence. Colloids and Surfaces B: Biointerfaces, 205, 111838.
Salmon, E., Sadzot, B., Maquet, P., Degueldre, C., Lemaire, C., Rigo, P., Comar, D., & Franck, G. (1994). Differential diagnosis of Alzheimer’s disease with PET. Journal of nuclear medicine: Official publication, Society of Nuclear Medicine, 35(3), 391–398.
Salve, P., Pise, S., & Bali, N. (2016). Formulation and Evaluation of Solid Lipid Nanoparticle Based Transdermal Drug Delivery System for Alzheimer’s Disease. Research Journal of Pharmaceutical Dosage Forms and Technology, 8(2), 73–80.
Schelterns, P., & Feldman, H. (2003). Treatment of Alzheimer’s disease; current status and new perspectives. The Lancet Neurology, 2(9), 539–547.
Schindler, S. E., Bollinger, J. G., Ovod, V., Mawuenyega, K. G., Li, Y., Gordon, B. A., Holtzman, D. M., Morris, J. C., Benzinger, T. L. S., & Xiong, C. (2019). High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology, 93(17), e1647–e1659.
Sharifi, S., Samani, A. A., Ahmadian, E., Eftekhari, A., Derakhshankhah, H., Jafari, S., Mokhtarpour, M., Vahed, S. Z., Salatin, S., & Dizaj, S. M. (2019). Oral delivery of proteins and peptides by mucoadhesive nanoparticles. Biointerface Research in Applied Chemistry, 9(2), 3849–3852.
Silveira M, Marques J (2010) Boosting Alzheimer disease diagnosis using PET images. In: 2010 20th International Conference on Pattern Recognition. IEEE, pp 2556–2559
Sivanesan S, Rajeshkumar S (2019) Gold nanoparticles in diagnosis and treatment of alzheimer’s disease. In: Nanobiotechnology in Neurodegenerative Diseases. Springer, pp 289–306
Sivasubramanian, M., Hsia, Y., & Lo, L. W. (2014). Nanoparticle-facilitated functional and molecular imaging for the early detection of cancer. Frontiers in Molecular Biosciences., 17(1), 15.
Sood, S., Jain, K., & Gowthamarajan, K. (2014). Intranasal therapeutic strategies for management of Alzheimer’s disease. Journal of drug targeting, 22(4), 279–294.
Špringer, T., Hemmerová, E., Finocchiaro, G., Krištofiková, Z., Vyhnálek, M., & Homola, J. (2020). Surface plasmon resonance biosensor for the detection of tau-amyloid β complex. Sensors and Actuators B: Chemical, 316, 128146.
Tan, M. S., Tan, L., Jiang, T., Zhu, X. C., Wang, H. F., Jia, C. D., & Yu, J. T. (2014). Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell death & disease, 5(8), e1382–e1382.
Thakur, G., Micic M., Yang Y., Li W., Movia D., Giordani S., Zhang H., Leblanc RM. (2011). Conjugated quantum dots inhibit the amyloid β (1–42) fibrillation process. International Journal of Alzheimer’s Disease. https://doi.org/10.4061/2011/502386
Thendral, V., Dharshni, T., Ramalakshmi, M., Girigoswami, A., & Girigoswami, K. (2019). Cerium oxide nanocluster based nanobiosensor for ROS detection. Biocatalysis and agricultural biotechnology, 19, 101124.
Tiwari, S., Atluri, V., Kaushik, A., Yndart, A., & Nair, M. (2019). Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. International journal of nanomedicine, 14, 5541.
Vakilinezhad, M. A., Amini, A., Javar, H. A., Zarandi, B. F. B. B., Montaseri, H., & Dinarvand, R. (2018). Nicotinamide loaded functionalized solid lipid nanoparticles improves cognition in Alzheimer’s disease animal model by reducing Tau hyperphosphorylation. DARU Journal of Pharmaceutical Sciences, 26(2), 165–177.
van Oostveen, W. M., & de Lange, E. (2021). Imaging techniques in Alzheimer’s disease: A review of applications in early diagnosis and longitudinal monitoring. International Journal of Molecular Sciences., 22(4), 2110.
Veerabhadrappa, B., Delaby, C., Hirtz, C., Vialaret, J., Alcolea, D., Lleó, A., Fortea, J., Santosh, M. S., Choubey, S., & Lehmann, S. (2020). Detection of amyloid beta peptides in body fluids for the diagnosis of alzheimer’s disease: Where do we stand? Critical reviews in clinical laboratory sciences, 57(2), 99–113.
Vieira, D. B., & Gamarra, L. F. (2016). Getting into the brain: Liposome-based strategies for effective drug delivery across the blood–brain barrier. International journal of nanomedicine, 11, 5381.
Vorobyov, V., Kaptsov, V., Gordon, R., Makarova, E., Podolski, I., & Sengpiel, F. (2015). Neuroprotective effects of hydrated fullerene C 60: Cortical and hippocampal eeg interplay in an amyloid-infused rat model of alzheimer’s disease. Journal of Alzheimer’s Disease, 45(1), 217–233.
Wadghiri, Y. Z., Sigurdsson, E. M., Sadowski, M., Elliott, J. I., Li, Y., Scholtzova, H., Tang, C. Y., Aguinaldo, G., Pappolla, M., & Duff, K. (2003). Detection of Alzheimer’s amyloid in transgenic mice using magnetic resonance microimaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 50(2), 293–302.
Xie, L., Luo, Y., Lin, D., Xi, W., Yang, X., & Wei, G. (2014). The molecular mechanism of fullerene-inhibited aggregation of Alzheimer’s β-amyloid peptide fragment. Nanoscale, 6(16), 9752–9762.
Xu, C., & Qu, X. (2014). Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia materials, 6(3), e90–e90.
Yang, Z.-Z., Zhang, Y.-Q., Wang, Z.-Z., Wu, K., Lou, J.-N., & Qi, X.-R. (2013). Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. International journal of pharmaceutics, 452(1–2), 344–354.
Yang, H. D., Kim, D. H., Lee, S. B., & Young, L. D. (2016). History of Alzheimer’s Disease. Dementia and neurocognitive disorders., 15(4), 115–121.
Youn, Y. C., Kang, S., Suh, J., Park, Y. H., Kang, M. J., Pyun, J.-M., Choi, S. H., Jeong, J. H., Park, K. W., & Lee, H.-W. (2019). Blood amyloid-β oligomerization associated with neurodegeneration of Alzheimer’s disease. Alzheimer’s research & therapy, 11(1), 40.
Zielińska, A., Carreiró, F., Oliveira, A. M., Neves, A., Pires, B., Venkatesh, D. N., Durazzo, A., Lucarini, M., Eder, P., & Silva, A. M. (2020). Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules, 25(16), 3731.
Zoltowska, K. M., & Berezovska, O. (2018). Dynamic nature of presenilin1/γ-secretase: Implication for Alzheimer’s disease pathogenesis. Molecular neurobiology, 55(3), 2275–2284.
Zrazhevskiy, P., Sena, M., & Gao, X. (2010). Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chemical Society Reviews, 39(11), 4326–4354.
Acknowledgements
We gratefully acknowledge the financial and infrastructural support from Chettinad Academy of Research and Education (CARE). GA thanks CARE for providing research fellowship as a JRF.
Funding
Council of Scientific and Industrial Research (CSIR), India (Scheme No. 01(2868)/ 17/EMR-II), INDIA, is acknowledged for financial support.
Author information
Authors and Affiliations
Contributions
NS and GA were involved in the compilation of literature and draft into a review paper. AG and KG participated in the supervision and incorporation of scientific views. AG and KG finalized the draft.
Corresponding author
Ethics declarations
Conflict of Interest
No
Research Involving Humans and Animals Statement
No
Informed Consent
No
Funding Statement
No
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Agraharam, G., Saravanan, N., Girigoswami, A. et al. Future of Alzheimer’s Disease: Nanotechnology-Based Diagnostics and Therapeutic Approach. BioNanoSci. 12, 1002–1017 (2022). https://doi.org/10.1007/s12668-022-00998-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-022-00998-8