Abstract
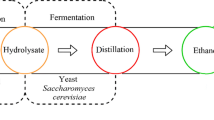
Acid and base saccharification (hydrolysis) are simple and direct ways to convert biomass into fermentable sugars to be used for the production of bioethanol as renewable liquid fuel. In this study, the marine green alga (Ulva fasciata) was converted to sugars by acid and base hydrolysis (Na OH, ammonium oxalate, HCL, and H2SO4) at different concentrations (1, 3, 5, 7 %) at 121 °C for one, two and three rounds in autoclave. Biological hydrolysis of alga was also done by bacterial growth and enzymatic hydrolysis of alga. The maximum amount of sugars was 700 ± 26.10 mg sugar/g alga biomass Ulva fasciata at one round of autoclave with 3 % sulphuric acid. The sugar ratio was 640.96 ± 9.6 mg sugar/g algal biomass Ulva fasciata that treated by a bacterial strain (Bacillus subtilis SH04). Sugar yields from Ulva fasciata by amylase partially purified from Bacillus subtilis SH04 (B4) and Bacillus cereus SH06 (B6) followed by autoclaving for one round gave 458.27 ± 6.55 and 516.07 ± 5.17 mg sugar/g algal biomass, respectively. The ethanol efficiency using Saccharomyces cerevisiae SH02 was 78.3 % ± 6 with 5 % sugar concentration that produced by acid hydrolysis. Ethanol production was 55.9 % ± 5.2 after enzymatic hydrolysis of alga and fermentation by Saccharomyces cerevisiae SH02.









Similar content being viewed by others
References
Abdel-Fattah AF, Edress M (1972) A study on the polysaccharide content of Ulva lactuca. Qualitas Plantarum Et Materiae Vegetabiles 6:15–22
Aehle W, Misset O (1999) Enzymes for industrial applications. In: Rehm HJ, Reed G (eds) Biotechnology, 2nd edn. Wiley-VCH, Germany
Aleem AA (1993) The marine algae of Alexandria (ED). Faculty of science. Alexandria university, Egypt
Aro N, Pakula T, Penttiillae M (2005) Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol Rev 29:719–739
Azad MA, Bae JH, Kim JS, Lim JK, Song KS, Shin BS, Kim HR (2009) Isolation and characterization of a novel thermo stable alpha-amylase from Korean pine seeds. N Biotechnol 26:143–149
Beveridge MCM, Sikdar PK, Frerichs GN, Millar S (1991) The ingestion of bacteria in suspension by the common carp Cyprinus carpio L. J of Fish Biology 39:825–831
Binder JB, Raines RT (2010) Fermentable sugars by chemical hydroysis of biomass. Proc Natl Acad Sci 107:4516–4521
Bisaria VS (1991) Bioprocessing of agro-residues to glucose and chemicals. In: Martin AM (ed) Elsevier, London pp 210–213
Bixler HJ, Porse H (2010) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Bryhmi E (1978) Quantitative differences between polysaccharide compositions in normal differentiated Ulva mutabilis undifferentiated mutant lumpy. Phycologia 17:119–124
Çaylak K, Sukan F (1998) Comparison of different production process for bioethanol. Turk J Chem 22(4):351–359
Charitha MD, Kumar MS (2012) Production, optimization and partial purification of cellulase by Aspergillus niger fermented with paper and timber sawmill industrial wastes. Microbiol Biotech Res 2:120–128
Devakia T, Sathivel A, Rao H, Raghavendranc B (2009) Stabilization of mitochondrial and microsomal function by polysaccharide of Ulva lactuca on d-Galactosamine induced hepatitis in rats. Chem Biol Interact 177:83–88
Dubois M, Gilles KA, Hamilton JK, PA Rebers, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Duke CS, Litaker W, Ramus J (1989) Effects of the temperature, nitrogen supply and tissue nitrogen on ammonium uptake rates of the Chlorophyte seaweeds Ulva curvata and Codium decorcatum. J Phycol 25:113–120
El-Sheekh MM, Hamouda RA (2016) Lipids extraction from the green alga Ankistrodesmus falcatus using different methods. Rend Fis Acc Lincei 1–7. doi:10.1007/s12210-016-0528-4
Fakroddin Md, Abdul Quayum Md, Monzurmorsned A, Naiyyum C (2012) Analysis of key factors affecting ethanol production by Saccharomyces cerevisiae IFST-072011. Biotechnology 11(4):248–252
Ferreira OF, Montijo NA, Martins ES, Mutton MJR (2015) Production of α-amylase by solid state fermentation by Rhizopus oryzae. Afr J Biotechnol 14(7):622–628
Foreman PK, Brown D, Dankmeyer L, Dean R, Diener S, Dunn Coleman NS, Goedegebuur F, Houfek TD, England GJ, Kelley AS, Meerman HJ, Mitchell T, Mitchinson C, Olivares HA, Teunissen PJM, Yao J, Ward M (2003) Transcriptional regulation of biomass degrading enzymes in the filamentous fungus Trichoderma. J Biol Chem 278:31988–31997
Fujita RM (1985) The role of nitrogen status in regulating transient ammonium uptake and nitrogen storage by macroalgae. J Exp Marine Biol Ecol 92:283–301
Govindaswamy S, Vane LM (2010) Multi-stage continuous culture fermentation of glucose–xylose mixtures to fuel ethanol using genetically engineered Saccharomyces cerevisiae 424S. Bioresour Technol 101:1277–1284
Gunasekaran P, Karunakaran T, Kasthuribal M (1986) Fermentation pattern of Zymomonas mobilis strains on different substrates a comparative study. J Biosci 10(2):181–186
Hamouda RA, Hussein MH, Hamza HA, Yeheia DS (2013) Production of bioethanol from macrogreen algae. Egypt journal of Botany. 3rd International Conference pp 235–246
Hamouda RA, Hussein MH, El El-Naggar N (2015) Potential value of red and brown seaweed for sustainable bioethanol production. Bangladesh J Bot 44:565–570
Jacob MB, Gerstein MJ (1960) Handbook of Microbiology. D. Van Nostrand Co., Inc., Princeton New Jersey
Kadar ZS, Thomsen AB (2010) Biofuel production from macroalage, poster presented at 32nd symposium on Biotechnology for fuels and chemicals, 19–22 April, 2010, FL, USA
Knoshaug EP, Darzins A (2011) Algal biofuels: the process. Chem Eng Progr 107:37–47
Krishnaveni S, Balasubramanian T, Sadasivam S (1984) Sugar distribution in sweet stalk sorghum. Food Chem 15:229–232
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Kumar CS, Ganesan P, Bhaskar N (2008) In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem 107:707–713
Kumar RS, Shankar T, Anandapandian KTK (2011) Characterization of alcohol resistant yeast Saccharomyces cerevisiae isolated from Toddy. Int Res J Microbiol 2(10):399–405
Lahaye M, Axelos MAV (1993) Gelling properties of water soluble polysaccharides from proliferating marine green seaweeds (Ulva spp.). Carbohydr Polym 22:261–265
Lahaye M, Kaeffer B (1997) Seaweed dietary fibres: structure, physico-chemical and biological properties relevant to intestinal physiology. Sciences des Aliments 17:563–584
Lee YY, Iyer P, Torget RW (1999) Dilute-acid hydrolysis of lignocellulosic biomass: recent progress in bioconversion of lignocellulosics. Adv Biochem Eng Biotechnol 65:93–115
Lenihan P, Orozco AO, Neill E, Ahmad MNM, Rooney DW, Walker GM (2010) Dilute acid hydrolysis of lignocellulosic biomass. Chem Eng J 156:395–403
Lin LL, Hsu WH (1997) A gene encoding for an alpha-amylase from thermophilic Bacillus sp. strain TS-23 and its expression in Escherichia coli. J Appl Microbiol 82:325–334
Lynd LR (1996) Overview and evaluation of fuel ethanol from cellulosic biomass: technology, economics, the environment, and policy. Annu Rev Energy Environ 21:403–465
Mata TM, Martins ANA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14:217. doi:10.1016/j.rser.2009.07.020
McHugh DJ (1987) Production and utilization of products from commercial seaweeds. FAO Fish Tech Paper 288:1–189
Morand P, Briand X (1996) Excessive growth of macroalgae: a symptom of environmental disturbance. Bot Mar 39:491–516
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Murata M, Nakazoe J (2001) Production and use of marine algae in Japan. Jarq-Jpn Agric Res Quart 35:281–290
Niranjane AJ, Madhou P, Stevenson TW (2007) The effect of carbohydrate carbon sources on the production of cellulase by Phlebia gigantean. Enzyme Microbial Technol 40:1464–1468
Nokov N, Marinova M, Dimitrova-Konaklieve S (1984) Chemical composition and biology of Black sea seaweed Ulva rigida Ag. Part 1. Formatriya (Sofia) 34:24–26
Pádua Md, Sérgio P, Fontoura G, Mathias AL (2004) Chemical Composition of Ulvaria oxysperma (Kützing) Bliding, Ulva lactuca (Linnaeus) and Ulva fasciata. Delile 47:49–55
Pengzhan Y, Quanbin Z, Ning L, Zuhong X, Yanmei W, Zhien L (2003) Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. Appl Phycol 15:21–27
Priest FG (1977) Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev 41:711–753
Priyadarshani I, Rath B (2012) Commercial and industrial applications of micro algae—A review. J Algal Biomass Utln 3(4):89–100
Pultz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Rainey FA, Ward-Rainey N, Kroppenstedt RM, Stackbrandt E (1996) The genus nocardiopsis represents a phylogenetically coherent taxon and a distinct Actinomycete Lineage: proDosal of Nocardiomaceae fam. nov. Int J Sys Bacteriol 46:1088–1092
Rajagopalan G, Krishnan C (2008) Alpha-amylase production from catabolite derepressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate. Bioresour Technol 99:3044–3050
Ross AB, Jones JM, Kubacki ML, Bridgeman T (2008) Classification of macroalgae as fuel and its thermochemical behavior. Bioresource Technol 99:6494–6504
Saha BC, Iten LB, Cotta MA, Wu YV (2005) Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Proc Biochem 40:3693–3700
Sanchez G, Pilcher L, Roslander C, Modig T, Galbe M, Liden G (2004) Dilute acid hydrolysis for fermentation of the Bolivian straw material Paja Brava. Bioresource Technol 93:249–256
Scott SA, Davey MP, Dennis JS, Horst I, Howe CJ, Lea-Smith DJ, Smith AG (2010) Biodiesel from algae: challenges and prospects. Curr Opin Biotechnol 21:277–286
Siddhanta AK, Goswami AM, Ramavat BK, Mody KH, Mairh OP (2001) Water soluble polysaccharides of marine algal species of Ulva (Ulvales, Chlorophyta) of Indian waters. Indian J of Mar Sci 30:166–172
Singh S, Kate BN, Banerjee UC (2005) Bioactive compounds from cyanobacteria and microalgae an overview. Crit Rev Biotechnol 25:73–95
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Taherzadeh MJ, Karimi K (2007) Enzyme-based hydrolysis processes for ethanol from lignocelluloses materials: a review. BioResources 2(4):707–738
Tahir A, Aftab M, Farasat T (2010) Effect of cultural conditions on ethanol production by locally isolated Saccharomyces cerevisiae BIO-07. J Appl Pharmacol 3:72–78
Taylor WS (1985) Marine algae of the eastern tropical and subtrobical coasts of Americas. ANN Arbor the university of Michigan press
Teather RM, Wood PJ (1982) Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43:777–780
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res 22:4673–4680
Thomsen AB, Arvaniti E, Xu J, Oleskowicz-Popiel P, Frenqvist T, Schultz-Jensen N, Kádár Z, Jensen M, Thomsen ST, Thygessen A (2010) Pretreatment technologies for production of 2G bioethanol from agricultural waste and crops. Speech at: 11th European Workshop on Lignocellulosic and Pulp, Hamburg, Germany, 16–19 August
Tseng CK (2001) Algal biotechnology industries and research activities in China. J Appl Phycol 13:375–380
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329:796–799
Wolfgang A (2007) Enzyme in industry: production and applications. Wiley-VCH, Weinheim
Xian Q, Lee YY, Pettersson PO, Torget RW (2003) Heterogeneous aspects of acid hydrolysis of alpha-cellulose. Appl Biochem and Biotech 105–108: 505–514. http://www.ncbi.nlm.nih.gov
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamouda, R.A., Sherif, S.A., Dawoud, G.T.M. et al. Enhancement of bioethanol production from Ulva fasciata by biological and chemical saccharification. Rend. Fis. Acc. Lincei 27, 665–672 (2016). https://doi.org/10.1007/s12210-016-0546-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-016-0546-2