Abstract
This study deals with the preparation and corrosion inhibition investigations of pyrazoline-sulfonamide hybrid, namely 4-((3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-4-yl)diazenyl) benzene sulfonamide 4. Elemental analysis, Fourier transform infrared spectroscopy, 1H nuclear magnetic resonance (1H NMR), and 13CNMR spectra investigated the pyrazoline-sulfonamide structure. The mild steel corrosion inhibition was investigated in 1 M HCl and 3.5% NaCl solutions using various techniques, including electrochemical, gravimetric, and microscopic techniques. The inhibition efficiency was 95.4 and 87.7% at 500 ppm inhibitor in 1 M HCl and 3.5% NaCl, respectively. The positive values of ∆Hads (44.184 and 31.403 kJ/mol, in 1 M HCl and 3.5% NaCl, respectively) indicate that inhibitor adsorption is an endothermic process. Moreover, the high values of Kads (0.281 and 0.148 M-1) indicate more significant adsorption and thus stronger inhibitory efficiency. Langmuir adsorption isotherm was used to evaluate mild steel corrosion inhibition by pyrazoline-sulfonamide. Experimental data agree with data obtained from B3LYP density functional theory calculations. Therefore, based on the experimental and theoretical data, pyrazoline-sulfonamide is recommended as a corrosion inhibitor of mild steel in aqueous media.
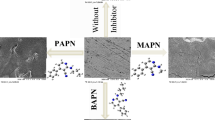
Graphical abstract
Pyrazoline-sulfonamide (PS) has been prepared and used as novel corrosion inhibitors of mild steel. Langmuir adsorption isotherm was used to evaluate MS corrosion inhibition by PS. Experimental data are in good agreement with B3LYP DFT calculations. Therefore, PS is recommended as a corrosion inhibitor of mild steel in aqueous media.







Similar content being viewed by others
References
Fateh A, Aliofkhazraei M and Rezvanian A 2020 Review of corrosive environments for copper and its corrosion inhibitors Arab. J. Chem. 13 481
Akdag A 2020 Electrochemical Synthesis and Corrosion Behaviour of Poly(o–anisidine)-TiO2 Nanocomposite on ZnNi-Plated Carbon Steel ChemistrySelect 5 2496
Hegde M B and Mohana K N 2020 A Sustainable and Eco-Friendly Polymer Based Graphene Oxide Nanocomposite Anti-Corrosion Coating on Mild Steel ChemistrySelect 5 1506
El-Haddad M N and Fouda AEAS 2021 Evaluation of Curam drug as an ecofriendly corrosion inhibitor for protection of stainless steel-304 in hydrochloric acid solution: Chemical, electrochemical, and surface morphology studies J. Chin. Chem. Soc. 68 826
Verma C, Ebenso E E, Bahadur I and Quraishi M A 2018 An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media J. Mol. Liq. 266 577
Singh P, Ebenso E E, Olasunkanmi L O, Obot I and Quraishi M 2016 Electrochemical, theoretical, and surface morphological studies of corrosion inhibition effect of green naphthyridine derivatives on mild steel in hydrochloric acid J. Phys. Chem. C 120 3408
Esmaeili N, Neshati J and Yavari I 2015 Corrosion inhibition of new thiocarbohydrazides on the carbon steel in hydrochloric acid solution J. Ind. Eng. Chem. 22 159
Abd E l-Lateef H M 2015 Experimental and computational investigation on the corrosion inhibition characteristics of mild steel by some novel synthesized imines in hydrochloric acid solutions Corros. Sci. 92 104
Branzoi F, Branzoi V and Licu C 2014 Corrosion inhibition of carbon steel in cooling water systems by new organic polymers as green inhibitors Mater. Corros. 65 637
Petrović M B, Simonović A T, Radovanović M B, Milić S M and Antonijević M M 2012 Influence of purine on copper behavior in neutral and alkaline sulfate solutions Chem. Pap. 66 664
Tasic Z Z and Antonijevic M M 2016 Copper corrosion behaviour in acidic sulphate media in the presence of 5-methyl-1 H-benzotriazole and 5-chloro-1 H-benzotriazole Chem. Pap. 70 620
Singh M B, Gabriel B I, Venkatraman M S, Cole I S, Moorthy C G and Emmanuel B 2022 Theory of impedance for initial corrosion of metals under a thin electrolyte layer: a coupled charge transfer-diffusion model J. Chem. Sci. 134 32
Emregül K C, Kurtaran R and Atakol O 2003 An investigation of chloride-substituted Schiff bases as corrosion inhibitors for steel Corros. Sci. 45 2803
Zarrouk A, Zarrok H, Ramli Y, Bouachrine M, Hammouti B, Sahibed-Dine A and Bentiss F 2016 Inhibitive properties, adsorption and theoretical study of 3, 7-dimethyl-1-(prop-2-yn-1-yl) quinoxalin-2 (1H)-one as efficient corrosion inhibitor for carbon steel in hydrochloric acid solution J. Mol. Liq. 222 239
Mahross M H, Taher M A, Mostfa MA, Chong K F and Ali G A M 2021 Experimental and quantum investigations of novel corrosion inhibitors based triazene derivatives for mild steel J. Mol. Struct. 1242 130831
Gaaz T S, Dakhil R M, Jamil D M, Al-Amiery A A, Kadhum A A and Takriff M 2020 Evaluation of green corrosion inhibition by extracts of citrus aurantium leaves against carbon steel in 1 M HCl medium complemented with quantum chemical assessment Int. J. Thin Film Sci. Technol. 9 171
Attar T, Nouali F, Kibou Z, Benchadli A, Messaoudi B, Choukchou-Braham E and Choukchou-Braham N 2021 Corrosion inhibition, adsorption and thermodynamic properties of 2-aminopyridine derivatives on the corrosion of carbon steel in sulfuric acid solution J. Chem. Sci. 133 109
El-Awady A A, Abd-El-Nabey B A and Aziz S G 1992 Kinetic-thermodynamic and adsorption isotherms analyses for the inhibition of the acid corrosion of steel by cyclic and open-chain amines J. Electrochem. Soc. 139 2149
Mostfa M A, Gomaa H, Othman I M M and Ali G A M 2021 Experimental and theoretical studies of a novel synthesized azopyrazole-benzenesulfonamide derivative as an efficient corrosion inhibitor for mild steel J. Iran. Chem. Soc. 18 1231
Ateya B, E l-Anadouli B and E l-Nizamy F 1984 The adsorption of thiourea on mild steel Corros. Sci. 24 509
Nehra B, Rulhania S, Jaiswal S, Kumar B, Singh G and Monga V 2020 Recent advancements in the development of bioactive pyrazoline derivatives Eur. J. Med. Chem. 205 112666
Ozmen Ozgun D, Gul H I, Yamali C, Sakagami H, Gulcin I, Sukuroglu M and Supuran CT 2019 Synthesis and bioactivities of pyrazoline benzensulfonamides as carbonic anhydrase and acetylcholinesterase inhibitors with low cytotoxicity Bioorg. Chem. 84 511
Sayed A M, Taher F A, Abdel-Samad M R, E l-Gaby MS, E l-Adl K and Saleh N M 2021 Design, synthesis, molecular docking, in silico ADMET profile and anticancer evaluations of sulfonamide endowed with hydrazone-coupled derivatives as VEGFR-2 inhibitors Bioorg. Chem. 108 104669
Bhandari S, Tripathi A and Saraf S 2013 Synthesis and anticonvulsant activity of some 2-pyrazoline derivatives Med. Chem. Res. 22 5290
Kharbanda C, Alam M S, Hamid H, Javed K, Bano S, Dhulap A, et al. 2014 Synthesis and evaluation of pyrazolines bearing benzothiazole as anti-inflammatory agents Bioorg. Med. Chem. 22 5804
Joshi S D, Dixit S R, Kirankumar M, Aminabhavi T M, Raju K, Narayan R, et al. 2016 Synthesis, antimycobacterial screening and ligand-based molecular docking studies on novel pyrrole derivatives bearing pyrazoline, isoxazole and phenyl thiourea moieties Eur. J. Med. Chem. 107 133
Kaplancıklı Z A, Özdemir A, Turan-Zitouni G, Altıntop M D and Can Ö D 2010 New pyrazoline derivatives and their antidepressant activity Eur. J. Med. Chem. 45 4383
Shamsuzzaman Khanam H, Dar A M, Siddiqui N and Rehman S 2016 Synthesis, characterization, antimicrobial and anticancer studies of new steroidal pyrazolines J. Saudi Chem. Soc. 20 7
Özkay Ü D, Can Ö D and Kaplancıklı Z A 2012 Antinociceptive activities of some triazole and pyrazoline moieties-bearing compounds Med. Chem. Res. 21 1056
Acharya B N, Saraswat D, Tiwari M, Shrivastava A K, Ghorpade R, Bapna S and Kaushik M P 2010 Synthesis and antimalarial evaluation of 1, 3, 5-trisubstituted pyrazolines Eur. J. Med. Chem. 45 430
Özdemir A, Turan-Zitouni G, Asım Kaplancıklı Z, Revial G, Demirci F and İşcan G 2010 Preparation of some pyrazoline derivatives and evaluation of their antifungal activities J. Enzyme Inhib. Med. Chem. 25 565
Bhat A R, Athar F and Azam A 2009 Bis-pyrazolines: synthesis, characterization and antiamoebic activity as inhibitors of growth of Entamoeba histolytica Eur. J. Med. Chem. 44 426
Mostafa M, Ashmawy A M, Reheim M A A, Bedair M A and Abuelela A M 2021 Molecular structure aspects and molecular reactivity of some triazole derivatives for corrosion inhibition of aluminum in 1 M HCl solution J. Mol. Struct. 1236 130292
Sehmi A, Ouici H B, Guendouzi A, Ferhat M, Benali O and Boudjellal F 2020 Corrosion Inhibition of Mild Steel by newly Synthesized Pyrazole Carboxamide Derivatives in HCl Acid Medium: Experimental and Theoretical Studies J. Electrochem. Soc. 167 155508
Murulana L C, Kabanda M M and Ebenso E E 2016 Investigation of the adsorption characteristics of some selected sulphonamide derivatives as corrosion inhibitors at mild steel/hydrochloric acid interface: Experimental, quantum chemical and QSAR studies J. Mol. Liq. 215 763
Ebenso E E, Arslan T, Kandemïrlı F, Love I, Öğretır CL, Saracoğlu M and Umoren S A 2010 Theoretical studies of some sulphonamides as corrosion inhibitors for mild steel in acidic medium Int. J. Quant. Chem. 110 614
Obot I and Obi-Egbedi N 2010 Indeno-1-one [2, 3-b] quinoxaline as an effective inhibitor for the corrosion of mild steel in 0.5 M H2SO4 solution Mater. Chem. Phys. 122 325
Shukla S N, Gaur P and Chaurasia B 2019 Studies on heterocyclic anchored Cu (II) complexes with ONO pincer type donor ligand as an efficient biomimetic and anticorrosion agent J. Mol. Struct. 1186 345
Kaur G, Utreja D, Jain N and Dhillon N 2020 Synthesis and Evaluation of Pyrazole Derivatives as Potent Antinemic Agents Russ. J. Org. Chem. 56 113
Subudhi B and Ghosh G 2012 Synthesis and antibacterial activity of some heterocyclic derivatives of sulfanilamide Bull. Chem. Soc. Ethiop. 26 455
Cheng Y-F, Du W-B, Liu K, Fu J-J, Wang Z-H, Li S-B and Fu J-L 2020 Mechanical properties and corrosion behaviors of Mg−4Zn−0.2Mn−0.2Ca alloy after long term in vitro degradation Trans. Nonfer. Metal. Soc. China 30 363
Idris A M, Suleiman M H A and Ali I H 2019 Evaluation of Leaf and Bark Extracts of Acacia tortilis as Corrosion Inhibitors for Mild Steel in Seawater: Experimental and Studies Int. J. Electrochem. Sci. 14 6406
Yadav M, Kumar S, Bahadur I and Ramjugernath D 2014 Corrosion inhibitive effect of synthesized thiourea derivatives on mild steel in a 15% HCl solution Int. J. Electrochem. Sci. 9 6529
Szklarska-Smialowska Z and Mankowski J 1978 Crevice corrosion of stainless steels in sodium chloride solution Corros. Sci. 18 953
Fragoza-Mar L, Olivares-Xometl O, Domínguez-Aguilar M A, Flores E A, Arellanes-Lozada P and Jiménez-Cruz F 2012 Corrosion inhibitor activity of 1, 3-diketone malonates for mild steel in aqueous hydrochloric acid solution Corros. Sci. 61 171
Hsissou R, Abbout S, Seghiri R, Rehioui M, Berisha A, Erramli H, et al. 2020 Evaluation of corrosion inhibition performance of phosphorus polymer for carbon steel in [1 M] HCl: Computational studies (DFT, MC and MD simulations) J. Mater. Res. Technol. 9 2691
Aslam J, Aslam R, Alrefaee S H, Mobin M, Aslam A, Parveen M and Mustansar Hussain C 2020 Gravimetric, electrochemical, and morphological studies of an isoxazole derivative as corrosion inhibitor for mild steel in 1M HCl Arab. J. Chem. 13 7744
Akinbulumo O A, Odejobi O J and Odekanle E L 2020 Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl Results Mater. 5 100074
Noor E A 2007 Temperature effects on the corrosion inhibition of mild steel in acidic solutions by aqueous extract of fenugreek leaves Int. J. Electrochem. Sci. 2 996
Abbout S, Hsissou R, Erramli H, Chabebe D, Salim R, Kaya S and Hajjaji N 2021 Gravimetric, electrochemical and theoretical study, and surface analysis of novel epoxy resin as corrosion inhibitor of carbon steel in 0.5 M H2SO4 solution J. Mol. Struct. 1245
Gouda G A H, Ali G A M and Seaf Elnasr T A 2015 Stability studies of selected metal Ions chelates with 2-(4-amino-1,5-dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-ylideneamino) phenol Int. J. Nanomater. Chem. 1 39
Deyab M, Ashmawy A M, Nessim M and Mohsen Q 2021 New Gemini surfactants based on alkyl benzenaminium: Synthesis and links to application of corrosion protection J. Mol. Liq. 332 115855
Ibrahim MAM, Korablov SF and Yoshimura M 2002 Corrosion of stainless steel coated with TiN, (TiAl)N and CrN in aqueous environments Corros. Sci. 44 815
Fiala A, Chibani A, Darchen A, Boulkamh A and Djebbar K 2007 Investigations of the inhibition of copper corrosion in nitric acid solutions by ketene dithioacetal derivatives Appl. Surf. Sci. 253 9347
Bosch R, Hubrecht J, Bogaerts W and Syrett B 2001 Electrochemical frequency modulation: a new electrochemical technique for online corrosion monitoring Corrosion 57 60
Ebenso E E, Arslan T, Kandemirli F, Caner N and Love I 2010 Quantum chemical studies of some rhodanine azosulpha drugs as corrosion inhibitors for mild steel in acidic medium Int. J. Quant. Chem. 110 1003
Chen S, He B, Liu Y, Wang Y and Zhu J 2014 Quantum chemical study of some benzimidazole and its derivatives as corrosion ınhibitors of steel in HCl solution Int. J. Electrochem. Sci. 9 5400
Frisch M, Trucks G, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B and Petersson G 2009 Gaussian 09, Revision d. 01, Gaussian Inc., Wallingford CT 201
Geerlings P, De Proft F and Langenaeker W 2003 Conceptual density functional theory Chem. Rev. 103 1793
Hsissou R, Abbout S, Safi Z, Benhiba F, Wazzan N, Guo L, et al. 2021 Synthesis and anticorrosive properties of epoxy polymer for CS in [1 M] HCl solution: Electrochemical, AFM, DFT and MD simulations Constr. Build. Mater. 270 121454
Lgaz H, Salghi R, Chaouiki A, Shubhalaxmi Jodeh S and Subrahmanya Bhat K 2018 Pyrazoline derivatives as possible corrosion inhibitors for mild steel in acidic media: A combined experimental and theoretical approach Cogent Eng. 5 1441585
Sayed A M, Saleh N M, El-Gaby M S A, Abdel-Samad M R K and Taher F A 2021 Synthesis and in vivo evaluation of novel benzimidazole-sulfonamide hybrids and Lucilia cuprina maggots’ excretion/secretion topical gels for wound healing J. Chin. Chem. Soc. 68 1291
Bedair M A, Soliman S A, Bakr MF, Gad E S, Lgaz H, Chung I-M, et al. 2020 Benzidine-based Schiff base compounds for employing as corrosion inhibitors for carbon steel in 1.0 M HCl aqueous media by chemical, electrochemical and computational methods J. Mol. Liq. 317 114015
Yilmaz N, Fitoz A, Ergun Ÿ and Emregül K C 2016 A combined electrochemical and theoretical study into the effect of 2-((thiazole-2-ylimino)methyl)phenol as a corrosion inhibitor for mild steel in a highly acidic environment Corros. Sci. 111 110
Hsissou R, Benhiba F, Dagdag O, El Bouchti M, Nouneh K, Assouag M, et al. 2020 Development and potential performance of prepolymer in corrosion inhibition for carbon steel in 1.0 M HCl: Outlooks from experimental and computational investigations J. Colloid Interface Sci. 574 43
Verma C, Quraishi M, Olasunkanmi L and Ebenso E E 2015 L-Proline-promoted synthesis of 2-amino-4-arylquinoline-3-carbonitriles as sustainable corrosion inhibitors for mild steel in 1 M HCl: experimental and computational studies RSC Adv. 5 85417
Yadav M, Sinha R, Kumar S, Bahadur I and Ebenso E 2015 Synthesis and application of new acetohydrazide derivatives as a corrosion inhibition of mild steel in acidic medium: Insight from electrochemical and theoretical studies J. Mol. Liq. 208 322
Noor E A 2009 Potential of aqueous extract of Hibiscus sabdariffa leaves for inhibiting the corrosion of aluminum in alkaline solutions J. Appl. Electrochem. 39 1465
Şahin M, Bilgic S and Yılmaz H 2002 The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl mediums Appl. Surf. Sci. 195 1
Ateya B, El-Anadouli B and El-Nizamy F 1984 The effect of thiourea on the corrosion kinetics of mild steel in H2SO4 Corros. Sci. 24 497
Ashmawy A M and Mostfa M 2021 Study of Eco-Friendly Corrosion Inhibition for Mild Steel in Acidic Environment Egypt J. Chem. 64 1285
Abbas M A, Eid A M, Abdou M M, Elgendy A, El-Saeed R A and Zaki E G 2021 Multifunctional Aspects of the Synthesized Pyrazoline Derivatives for AP1 5L X60 Steel Protection Against MIC and Acidization: Electrochemical: In Silico, and SRB Insights ACS Omega 6 8894
Lgaz H, Saha S K, Chaouiki A, Bhat K S, Salghi R, Shubhalaxmi Banerjee P, et al. 2020 Exploring the potential role of pyrazoline derivatives in corrosion inhibition of mild steel in hydrochloric acid solution: Insights from experimental and computational studies Constr. Build. Mater. 233 117320
Ali G A M, Yusoff MM, Shaaban E R and Chong K F 2017 High performance MnO2 nanoflower supercapacitor electrode by electrochemical recycling of spent batteries Ceram. Int. 43 8440
El-Rabiee M M, Helal N H, El-Hafez G M A and Badawy W A 2008 Corrosion control of vanadium in aqueous solutions by amino acids J. Alloys Compd. 459 466
Deghadi R G, Elsharkawy A E, Ashmawy A M and Mohamed G G 2021 Can One Novel Series of Transition Metal Complexes of Oxy-dianiline Schiff Base Afford Advances in Both Biological Inorganic Chemistry and Materials Science? Comments Inorg. Chem. 1
Abdel-Rehim S, Khaled K and Abd-Elshafi N 2006 Electrochemical frequency modulation as a new technique for monitoring corrosion inhibition of iron in acid media by new thiourea derivative Electrochim. Acta 51 3269
Hegazy M, Hasan AM, Emara M, Bakr MF and Youssef AH 2012 Evaluating four synthesized Schiff bases as corrosion inhibitors on the carbon steel in 1 M hydrochloric acid Corros. Sci. 65 67
Abu Bakar N H, Ali G A M, Ismail J, Algarni H and Chong K F 2019 Size-dependent corrosion behavior of graphene oxide coating Prog. Org. Coat. 134 272
Li X, Deng S and Fu H 2011 Triazolyl blue tetrazolium bromide as a novel corrosion inhibitor for steel in HCl and H2SO4 solutions Corros. Sci. 53 302
Deng S, Li X and Fu H 2011 Alizarin violet 3B as a novel corrosion inhibitor for steel in HCl, H2SO4 solutions Corros. Sci. 53 3596
Ansari K and Quraishi M 2014 Bis-Schiff bases of isatin as new and environmentally benign corrosion inhibitor for mild steel J. Ind. Eng. Chem. 20 2819
Obi-Egbedi N and Obot I 2011 Inhibitive properties, thermodynamic and quantum chemical studies of alloxazine on mild steel corrosion in H2SO4 Corros. Sci. 53 263
Ashmawy A M, El-Sawy A M, Ali A A, El-Bahy S M and Alahl A A S 2021 Oxidative stability performance of new azophenol derivatives as antioxidants in working fluids for high-temperature solar applications Sol. Energy Mater. Sol. Cells 230 111282
Yadav M, Kumar S, Purkait T, Olasunkanmi L, Bahadur I and Ebenso E 2016 Electrochemical, thermodynamic and quantum chemical studies of synthesized benzimidazole derivatives as corrosion inhibitors for N80 steel in hydrochloric acid J. Mol. Liq. 213 122
Obot I, Macdonald D and Gasem Z 2015 Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: an overview Corros. Sci. 99 1
Mohsenifar F, Jafari H and Sayin K 2016 Investigation of thermodynamic parameters for steel corrosion in acidic solution in the presence of N, N′-Bis (phloroacetophenone)-1, 2 propanediamine J. Bio-Tribo-Corros. 2 1
Kathirvel K, Thirumalairaj B and Jaganathan M 2014 Quantum chemical studies on the corrosion inhibition of mild steel by piperidin-4-one derivatives in 1 M H3PO4 Open J. Met. 4 73
Behpour M, Ghoreishi S, Soltani N and Salavati-Niasari M 2009 The inhibitive effect of some bis-N, S-bidentate Schiff bases on corrosion behaviour of 304 stainless steel in hydrochloric acid solution Corros. Sci. 51 1073
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kamal, AB., Mostfa, M.A., Ashmawy, A.M. et al. Corrosion inhibition behavior of the synthesized pyrazoline-sulfonamide hybrid of mild steel in aqueous solutions: experimental and quantum investigations. J Chem Sci 134, 90 (2022). https://doi.org/10.1007/s12039-022-02086-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-022-02086-6