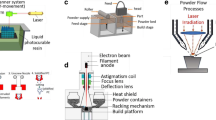
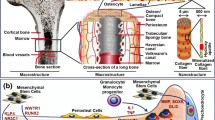
Abstract
Effective clinical methods for large bone defects are not yet available on account of the complex intrinsic structure and mechanical characteristics of natural bone tissue. It remains a challenge to restore bone damage to its original form by tissue engineering. With the continuous development of three-dimensional (3D) printing in recent years, the emergence of new technical supports and material innovations has established the foundation for bone tissue engineering (BTE). 3D printing has significant advantages for personalized treatment, as it allows for the specific fabrication of scaffolds with appropriate size, shape and intrinsic structural characteristics via patients’ computerized axial tomography scan or magnetic resonance imaging. In this review, we first systematically reviewed the development of 3D printing, printing methods and the selection of printing inks, then focused on the application of high-strength hydrogels in 3D printing for BTE. A brief anticipation of the future development of 3D printing was presented.
Graphical abstract






Similar content being viewed by others
References
Marrella A, Lee TY, Lee DH et al (2018) Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater Today 21(4):362–376. https://doi.org/10.1016/j.mattod.2017.10.005
Zhang T, Wei Q, Zhou H et al (2021) Three-dimensional-printed individualized porous implants: a new “implant-bone” interface fusion concept for large bone defect treatment. Bioactive Mater 6:3659–3670. https://doi.org/10.1016/j.bioactmat.2021.03.030
Nie L, Chen D, Suo J et al (2012) Physicochemical characterization and biocompatibility in vitro of biphasic calcium phosphate/polyvinyl alcohol scaffolds prepared by freeze-drying method for bone tissue engineering applications. colloids and surfaces. B Biointerfaces 100:169–176. https://doi.org/10.1016/j.colsurfb.2012.04.046
Zhang Z, Jia B, Han Y et al (2021) Biodegradable ZnLiCa ternary alloys for critical-sized bone defect regeneration at load-bearing sites: in vitro and in vivo studies. Bioactive Mater 6:3999–4013. https://doi.org/10.1016/j.bioactmat.2021.03.045
Swanson W, Zhang Z, Xiu K et al (2020) Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater 118:215–232. https://doi.org/10.1016/j.actbio.2020.09.052
Shi R, Huang Y, Ma C et al (2018) Current advances for bone regeneration based on tissue engineering strategies. Front Med. https://doi.org/10.1007/s11684-018-0629-9
Ratheesh G, Vaquette C, Xiao Y (2020) Patient‐specific bone particles bioprinting for bone tissue engineering. Adv Healthc Mater 9(23):2001323. https://doi.org/10.1002/adhm.202001323
Lin K-F, He S, Song Y et al (2016) Low-temperature additive manufacturing of biomimic three-dimensional hydroxyapatite/collagen scaffolds for bone regeneration. ACS Appl Mater Interfaces 8(11):6905–6916. https://doi.org/10.1021/acsami.6b00815
Jose M, Thomas V, Johnson K et al (2008) Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater 5:305–315. https://doi.org/10.1016/j.actbio.2008.07.019
Nakano T, Ishimoto T, Matsugaki A et al (2020) Control of crystallographic orientation by metal additive manufacturing process of β-type Ti alloys based on the bone tissue anisotropy. MATEC Web of Conf 321:05002. https://doi.org/10.1051/matecconf/202032105002
Schatkoski VM, doAmaralMontanheirodeMenezes TLBRC et al (2021) Current advances concerning the most cited metal ions doped bioceramics and silicate-based bioactive glasses for bone tissue engineering. Ceramics Int 47(3):2999–3012
Camara-Torres M, Duarte S, Sinha R et al (2021) 3D additive manufactured composite scaffolds with antibiotic-loaded lamellar fillers for bone infection prevention and tissue regeneration. Bioactive Mater 6(4):1073–1082. https://doi.org/10.1016/j.bioactmat.2020.09.031
Yinze X, Gao R-N, Zhang H et al (2020) Rationally designed functionally graded porous Ti6Al4V scaffolds with high strength and toughness built via selective laser melting for load-bearing orthopedic applications. J Mech Behav Biomed Mater 104:103673. https://doi.org/10.1016/j.jmbbm.2020.103673
Zhang H, Huang H, Hao G et al (2021) 3D Printing hydrogel scaffolds with nanohydroxyapatite gradient to effectively repair osteochondral defects in rats. Adv Funct Mater 31(1):2006697. https://doi.org/10.1002/adfm.202006697
Zhai X, Ma Y, Hou C et al (2017) 3D-printed high strength bioactive supramolecular polymer/clay nanocomposite hydrogel scaffold for bone regeneration. ACS Biomater Sci Eng. https://doi.org/10.1021/acsbiomaterials.7b00224
Wang X, Xu S, Zhou S et al (2016) Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: a review. Biomaterials 83:127–141. https://doi.org/10.1016/j.biomaterials.2016.01.012
Bai L, Zhao Y, Chen P et al (2021) Targeting early healing phase with titania nanotube arrays on tunable diameters to accelerate bone regeneration and osseointegration. Small 17(4):2006287. https://doi.org/10.1002/smll.202006287
Bai L, Liu Y, Du Z et al (2018) Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration. Acta Biomater 76:344–358. https://doi.org/10.1016/j.actbio.2018.06.023
Koons G, Diba M, Mikos A (2020) Materials design for bone-tissue engineering. Nat Rev Mater. https://doi.org/10.1038/s41578-020-0204-2
Wang Y, Huang X, Zhang X (2021) Ultrarobust, tough and highly stretchable self-healing materials based on cartilage-inspired noncovalent assembly nanostructure. Nature Commun. https://doi.org/10.1038/s41467-021-21577-7
Hua M, Shuwang W, Ma Y et al (2021) Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 590(7847):594–599. https://doi.org/10.1038/s41586-021-03212-z
Hua J, Ng PF, Fei B (2018) High-strength hydrogels: microstructure design, characterization and applications: high-strength hydrogels: a review of microstructure design, characterization and applications. J Polym Sci, Part B: Polym Phys 56:1325–1335. https://doi.org/10.1002/polb.24725
Hirsch M, Charlet A, Amstad E (2020) 3D printing of strong and tough double network granular hydrogels. Adv Func Mater. https://doi.org/10.1002/adfm.202005929
Manavitehrani I, Le TYL, Daly S et al (2018) Formation of porous biodegradable scaffolds based on poly(propylene carbonate) using gas foaming technology. Mater Sci Eng, C. https://doi.org/10.1016/j.msec.2018.11.088
Yao H, Kang J, Li W et al (2017) Novel β-TCP/PVA bilayered hydrogels with considerable physical and bio-functional properties for osteochondral repair. Biomed Mater. https://doi.org/10.1088/1748-605X/aa8541
Nahanmoghadam A, Asemani M, Gooadrzi V et al (2020) Design and fabrication of bone tissue scaffolds based on pcl/phbvcontaining hydroxyapatite nanoparticles: dual-leaching technique. J Biomed Mater Res, Part A. https://doi.org/10.1002/jbm.a.37087
Lan W, Zhang X, Xu M et al (2019) Carbon nanotube reinforced polyvinyl alcohol/biphasic calcium phosphate scaffold for bone tissue engineering. RSC Adv 9:38998–39010. https://doi.org/10.1039/C9RA08569F
Kazimierczak P, Benko A, Pałka K et al (2020) Novel synthesis method combining a foaming agent with freeze-drying to obtain hybrid highly macroporous bone scaffolds. J Mater Sci Technol. https://doi.org/10.1016/j.jmst.2020.01.006
Bandyopadhyay A, Mitra I, Bose S (2020) 3D printing for bone regeneration. Curr Osteoporos Rep 18:1–10. https://doi.org/10.1007/s11914-020-00606-2
Han X, Sun M, Chen B et al (2021) Lotus seedpod-inspired internal vascularized 3D printed scaffold for bone tissue repair. Bioactive Mater 6(6):1639–1652. https://doi.org/10.1016/j.bioactmat.2020.11.019
Bendtsen ST, Quinnell SP, Wei M (2017) Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J Biomed Mater Res Part A 105(5):1457–1468. https://doi.org/10.1002/jbm.a.36036
Wang C, Huang W, Zhou Y et al (2020) 3D printing of bone tissue engineering scaffolds. Bioactive Mater. https://doi.org/10.1016/j.bioactmat.2020.01.004
Maroulakos M, Kamperos G, Tayebi L et al (2018) Applications of 3D printing on craniofacial bone repair: a systematic review. J Dent. https://doi.org/10.1016/j.jdent.2018.11.004
Lipian M, Kulak M, Stepien M (2019) Fast track integration of computational methods with experiments in small wind turbine development. Energies 12(9):1625. https://doi.org/10.3390/en12091625
Zuo H, Liu Z, Zhang L et al (2021) Self-healing materials enable free-standing seamless large-scale 3D printing. Sci China-Mater. https://doi.org/10.1007/s40843-020-1603-y
Pasricha A, Greeninger R (2018) Exploration of 3D printing to create zero-waste sustainable fashion notions and jewelry. Fashion Text. https://doi.org/10.1186/s40691-018-0152-2
Kelly C, Miller A, Hollister S et al (2017) Design and structure-function characterization of 3d printed synthetic porous biomaterials for tissue engineering. Adv Healthcare Mater. https://doi.org/10.1002/adhm.201701095
Tay YWD, Panda B, Paul SC et al (2017) 3D printing trends in building and construction industry: a review. Virtual Phys Prototyp 12(3):261–276. https://doi.org/10.1080/17452759.2017.1326724
Yang Y, Zhang Q, Xu T et al (2020) Photocrosslinkable nanocomposite ink for printing strong, biodegradable and bioactive bone graft. Biomaterials. https://doi.org/10.1016/j.biomaterials.2020.120378
Daly AC, Cunniffe GM, Sathy BN et al (2016) 3D Bioprinting of developmentally inspired templates for whole bone organ engineering. Adv Healthcare Mater 5(18):2353–2362. https://doi.org/10.1002/adhm.201600182
Palmieri V, Lattanzi W, Perini G et al (2020) 3D-printed graphene for bone reconstruction. 2D Mater 7(2):022004. https://doi.org/10.1088/2053-1583/ab6a5d
Feng Z, Li Y, Hao L et al (2019) Graphene-reinforced biodegradable resin composites for stereolithographic 3d printing of bone structure scaffolds. J Nanomater 2019:1–13. https://doi.org/10.1155/2019/9710264
Xi L, Zhang Y, Gupta H et al (2020) A multiscale study of structural and compositional changes in a natural nanocomposite: Osteoporotic bone with chronic endogenous steroid excess. Bone 143:115666. https://doi.org/10.1016/j.bone.2020.115666
Midha S, Dalela M, Sybil D et al (2019) Advances in three-dimensional bioprinting of bone: Progress and challenges. J Tissue Eng Regen Med 13(6):925–945. https://doi.org/10.1002/term.2847
Tang A, Ji J, Li J et al (2021) Nanocellulose/pegda aerogels with tunable poisson’s ratio fabricated by stereolithography for mouse bone marrow mesenchymal stem cell culture. Nanomaterials 11(3):603. https://doi.org/10.3390/nano11030603
van Bochove B, Grijpma DW (2019) Photo-crosslinked synthetic biodegradable polymer networks for biomedical applications. J Biomater Sci-Polym Ed 30(2):77–106. https://doi.org/10.1080/09205063.2018.1553105
Wei Y, Zhao D, Cao Q et al (2020) Stereolithography-based additive manufacturing of high performance osteoinductive calcium phosphate ceramics by a digital light-processing system. ACS Biomater Sci Eng 6(3):1787–1797. https://doi.org/10.1021/acsbiomaterials.9b01663
Chen Y, Furukawa T, Ibi T et al (2021) Multi-scale micro-stereolithography using optical fibers with a photocurable ceramic slurry. Optical Mater Express 11(1):105–114. https://doi.org/10.1364/ome.404217
ZhouFuHe L‐YJY (2020) A review of 3D printing technologies for soft polymer materials. Adv Functional Mater 30(28):2000187. https://doi.org/10.1002/adfm.202000187
Heinrich MA, Liu W, Jimenez A et al (2019) 3D Bioprinting: from benches to translational applications. Small. https://doi.org/10.1002/smll.201805510
Anandakrishnan N, Ye H, Guo Z et al (2021) Fast stereolithography printing of large-scale biocompatible hydrogel models. Adv Healthc Mater 10(10):e2002103. https://doi.org/10.1002/adhm.202002103
Safonov A, Maltsev E, Chugunov S et al (2020) Design and fabrication of complex-shaped ceramic bone implants via 3d printing based on laser stereolithography. Appl Sci 10(20):7138
Le Guehennec L, Dorien VH, Plougonven E et al (2020) In vitro and in vivo biocompatibility of calcium-phosphate scaffolds three-dimensional printed by stereolithography for bone regeneration. J Biomed Mater Res, Part A 108(3):412–425. https://doi.org/10.1002/jbm.a.36823
Amler AK, Dinkelborg PH, Schlauch D et al (2021) Comparison of the translational potential of human mesenchymal progenitor cells from different bone entities for autologous 3d bioprinted bone grafts. Int J Mol Sci 22(2):796. https://doi.org/10.3390/ijms22020796
Thavasiappan K, Venkatesan MS, Ariffuddeen M et al (2020) Design, analysis, fabrication and testing of PC porous scaffolds using rapid prototyping in clinical applications. Biomedicine 39(2):339–345. https://doi.org/10.51248/.v39i2.204
Chimene D, Kaunas R, Gaharwar AK (2020) Hydrogel bioink reinforcement for additive manufacturing: a focused review of emerging strategies. Adv Mater 32(1):1902026. https://doi.org/10.1002/adma.201902026
Tromans G (2006) Rapid manufacturing: an industrial revolution for the digital age, pp 211–219.
Truby RL, Lewis JA (2016) Printing soft matter in three dimensions. Nature 540(7633):371–378. https://doi.org/10.1038/nature21003
Nadgorny M, Ameli A (2018) Functional polymers and nanocomposites for 3d printing of smart structures and devices. ACS Appl Mater Interfaces 10(21):17489–17507. https://doi.org/10.1021/acsami.8b01786
Yang K, Grant JC, Lamey P et al (2017) Diels–alder reversible thermoset 3d printing: isotropic thermoset polymers via fused filament fabrication. Adv Functional Mater 27(24):1700318. https://doi.org/10.1002/adfm.201700318
Nowicki MA, Castro NJ, Plesniak MW et al (2016) 3D printing of novel osteochondral scaffolds with graded microstructure. Nanotechnology 27(41):414001. https://doi.org/10.1088/0957-4484/27/41/414001
Alizadeh-Osgouei M, Li Y, Vahid A et al (2020) High strength porous PLA gyroid scaffolds manufactured via fused deposition modeling for tissue-engineering applications. Smart Mater Med 2:15–25. https://doi.org/10.1016/j.smaim.2020.10.003
Chen G, Chen N, Wang Q (2019) Fabrication and properties of poly(vinyl alcohol)/β-tricalcium phosphate composite scaffolds via fused deposition modeling for bone tissue engineering. Compos Sci Technol 172:17–28. https://doi.org/10.1016/j.compscitech.2019.01.004
Distler T, Fournier N, Gruenewald A et al (2020) Polymer-bioactive glass composite filaments for 3d scaffold manufacturing by fused deposition modeling: fabrication and characterization. Front Bioengin Biotechnol 8:552. https://doi.org/10.3389/fbioe.2020.00552
Yang C, Li J, Chongzun Z et al (2019) advanced antibacterial activity of biocompatible tantalum nanofilm via enhanced local innate immunity. Acta Biomater. https://doi.org/10.1016/j.actbio.2019.03.027
Nulty J, Freeman FE, Browe DC et al (2021) 3D bioprinting of prevascularised implants for the repair of critically-sized bone defects. Acta Biomater 126:154–169. https://doi.org/10.1016/j.actbio.2021.03.003
Ojansivu M, Rashad A, Ahlinder A et al (2019) Wood-based nanocellulose and bioactive glass modified gelatin-alginate bioinks for 3D bioprinting of bone cells. Biofabrication 11(3):035010. https://doi.org/10.1088/1758-5090/ab0692
Mandrycky C, Wang Z, Kim K et al (2016) 3D bioprinting for engineering complex tissues. Biotechnol Adv 34(4):422–434. https://doi.org/10.1016/j.biotechadv.2015.12.011
Okafor-Muo OL, Hassanin H, Kayyali R et al (2020) 3D Printing of solid oral dosage forms: numerous challenges with unique opportunities. J Pharm Sci 109(12):3535–3550. https://doi.org/10.1016/j.xphs.2020.08.029
Lv C, Zhu L, Shi J et al (2018) The fabrication of tissue engineering scaffolds by inkjet printing technology. Mater Sci Forum 934:129–133. https://doi.org/10.4028/www.scientific.net/MSF.934.129
Rajzer I, Rom M, Menaszek E et al (2015) Conductive PANI patterns on electrospun PCL/gelatin scaffolds modified with bioactive particles for bone tissue engineering. Mater Lett 138:60–63. https://doi.org/10.1016/j.matlet.2014.09.077
Gao G, Schilling AF, Yonezawa T et al (2014) Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol J 9(10):1304–1311. https://doi.org/10.1002/biot.201400305
Cui X, Dean D, Ruggeri ZM et al (2010) Cell damage evaluation of thermal inkjet printed chinese hamster ovary cells. Biotechnol Bioeng 106(6):963–969. https://doi.org/10.1002/bit.22762
Vanderburgh JP, Fernando SJ, Merkel AR et al (2017) Fabrication of trabecular bone-templated tissue-engineered constructs by 3d inkjet printing. Adv Healthcare Mater 6(22):1700369. https://doi.org/10.1002/adhm.201700369
Barui S, Panda AK, Naskar S et al (2019) 3D inkjet printing of biomaterials with strength reliability and cytocompatibility: quantitative process strategy for Ti-6Al-4V. Biomaterials 213:119212. https://doi.org/10.1016/j.biomaterials.2019.05.023
Liao B, Xia RF, Li W et al (2021) 3D-Printed Ti6Al4V scaffolds with graded triply periodic minimal surface structure for bone tissue engineering. J Mater Eng Perform 30:4993–5004. https://doi.org/10.1007/s11665-021-05580-z
Kamboj N, Aghayan M, Sara Rodrigo-Vázquez C et al (2019) Novel silicon-wollastonite based scaffolds for bone tissue engineering produced by selective laser melting. Ceram Int. https://doi.org/10.1016/j.ceramint.2019.08.208
Hull SM, Lindsay CD, Brunel LG et al (2021) 3D Bioprinting using universal orthogonal network (UNION) bioinks. Adv Func Mater 31(7):2007983. https://doi.org/10.1002/adfm.202007983
Kim SH, Yeon YK, Lee JM et al (2018) Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat Commun 9:1620. https://doi.org/10.1038/s41467-018-04517-w
He Y, Wang F, Wang X et al (2021) A photocurable hybrid chitosan/acrylamide bioink for DLP based 3D bioprinting. Mater Des 202:109588. https://doi.org/10.1016/j.matdes.2021.109588
Hong H, Seo YB, Kim DY et al (2020) Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 232:119679. https://doi.org/10.1016/j.biomaterials.2019.119679
Ouyang L, Armstrong JPK, Lin Y et al (2020) Expanding and optimizing 3D bioprinting capabilities using complementary network bioinks. Sci Adv. https://doi.org/10.1126/sciadv.abc5529
Duymaz BT, Erdiler FB, Alan T et al (2019) 3D bio-printing of levan/polycaprolactone/gelatin blends for bone tissue engineering: characterization of the cellular behavior. Eur Polymer J 119:426–437. https://doi.org/10.1016/j.eurpolymj.2019.08.015
Micic M, Antonijevic D, Milutinovic-Smiljanic S et al (2020) Developing a novel resorptive hydroxyapatite-based bone substitute for over-critical size defect reconstruction: physicochemical and biological characterization and proof of concept in segmental rabbit’s ulna reconstruction. Biomed Eng Biomedizinische Technik 65(4):491–505. https://doi.org/10.1515/bmt-2019-0218
Demirtas TT, Irmak G, Gumusderelioglu M (2017) A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 9(3):035003. https://doi.org/10.1088/1758-5090/aa7b1d
Liu X, Gaihre B, George MN et al (2021) 3Dbioprinting of oligo (poly ethylene glycol fumarate) for bone and nerve tissue engineering. J Biomed Mater Res Part A 109(1):6–17. https://doi.org/10.1002/jbm.a.37002
Anada T, Pan C-C, Stahl AM et al (2019) Vascularized bone-mimetic hydrogel constructs by 3d bioprinting to promote osteogenesis and angiogenesis. Int J Mol Sci 20(5):1096. https://doi.org/10.3390/ijms20051096
Bose S, Vahabzadeh S, Bandyopadhyay A (2013) Bone tssue engineering using 3D printing. Mater Today 16:496–504. https://doi.org/10.1016/j.mattod.2013.11.017
Ardelean IL, Gudovan D, Ficai D et al (2018) Collagen/hydroxyapatite bone grafts manufactured by homogeneous/heterogeneous 3D printing. Mater Lett 231:179–182. https://doi.org/10.1016/j.matlet.2018.08.042
Aldana A, Valente F, Dilley R et al (2020) Development of 3D bioprinted GelMA-alginate hydrogels with tunable mechanical properties. Bioprinting 21:e00105. https://doi.org/10.1016/j.bprint.2020.e00105
Chimene D, Lennox KK, Kaunas RR et al (2016) Advanced bioinks for 3d printing: a materials science perspective. Ann Biomed Eng 44(6):2090–2102. https://doi.org/10.1007/s10439-016-1638-y
Yang Yang, Song Xuan, Li Xiangjia et al (2018) Recent progress in biomimetic additive manufacturing technology: from materials to functional structures. Adv Mater 30(36):1706539. https://doi.org/10.1002/adma.201706539
Ouyang L, Rui Yao Y, Zhao WS (2016) Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 8(3):035020. https://doi.org/10.1088/1758-5090/8/3/035020
Siu TL, Rogers JM, Lin K et al (2018) Custom-made titanium 3-dimensional printed interbody cages for treatment of osteoporotic fracture-related spinal deformity. World Neurosurg 111:1–5. https://doi.org/10.1016/j.wneu.2017.11.160
Nune KC, Misra RDK, Gaytan SM et al (2015) Interplay between cellular activity and three-dimensional scaffold-cell constructs with different foam structure processed by electron beam melting. J Biomed Mater Res Part A 103(5):1677–1692. https://doi.org/10.1002/jbm.a.35307
Yu W, Zhao H, Ding Z et al (2016) In vitro and in vivo evaluation of MgF2 coated AZ31 magnesium alloy porous scaffolds for bone regeneration. Colloids Surf B. https://doi.org/10.1016/j.colsurfb.2016.10.037
Dumas M, Terriault P, Brailovski V (2017) Modelling and characterization of a porosity graded lattice structure for additively manufactured biomaterials. Mater Des 121:383–392. https://doi.org/10.1016/j.matdes.2017.02.021
Zheng Y, Han Q, Wang J et al (2020) Promotion of osseointegration between Implant and bone interface by titanium alloy porous scaffolds prepared by 3D printing. ACS Biomater Sci Eng 6(9):5181–5190. https://doi.org/10.1021/acsbiomaterials.0c00662
Soro N, Attar H, Brodie E et al (2019) Evaluation of the mechanical compatibility of additively manufactured porous Ti–25Ta alloy for load-bearing implant applications. J Mech Behav Biomed Mater 97:149–158. https://doi.org/10.1016/j.jmbbm.2019.05.019
Kuo T-Y, Chin W-H, Chien C-S et al (2019) Mechanical and biological properties of graded porous tantalum coatings deposited on titanium alloy implants by vacuum plasma spraying. Surf Coat Technol 372:399–409. https://doi.org/10.1016/j.surfcoat.2019.05.003
Weng Z, Bai L, Liu Y et al (2019) Osteogenic activity, antibacterial ability, and Ni release of Mg-incorporated Ni-Ti-O nanopore coatings on NiTi alloy. Appl Surf Sci 486:441–451. https://doi.org/10.1016/j.apsusc.2019.04.259
Lee JW, Wen HB, Battula S et al (2015) Outcome after placement of tantalum porous engineered dental implants in fresh extraction sockets a canine study. Int J Oral Maxillofac Implants 30(1):134–142
Liu S, Hu X, Ma X et al (2016) Promotion of osteointegration under diabetic conditions by tantalum coating-based surface modification on 3-dimensional printed porous titanium implants. Colloids Surf B. https://doi.org/10.1016/j.colsurfb.2016.09.018
Bandyopadhyay A, Mitra I, Shivaram A et al (2019) Direct comparison of additively manufactured porous titanium and tantalum implants towards in vivo osseointegration. Addit Manuf 28:259–266. https://doi.org/10.1016/j.addma.2019.04.025
Zhao DW, Ma ZJ, Wang TN et al (2019) Biocompatible porous tantalum metal plates in the treatment of tibial fracture. Orthop Surg 11(2):325–329. https://doi.org/10.1111/os.12432
Zhao G, Li S, Chen X et al (2019) Porous tantalum scaffold fabricated by gel casting based on 3D printing and electrolysis. Mater Lett 239:5–8. https://doi.org/10.1016/j.matlet.2018.12.047
Xiong Z, Liu W, Qian H et al (2021) Tantalum nanoparticles reinforced PCL scaffolds using direct 3D printing for bone tissue engineering. Front Mater 8(10):3389. https://doi.org/10.3389/fmats.2021.609779
Li Y, Zhou J, Pavanram P et al (2018) Additively manufactured biodegradable porous magnesium. Acta Biomater 67:378–392. https://doi.org/10.1016/j.actbio.2017.12.008
Zhao S, Xie K, Guo Y et al (2020) Fabrication and biological activity of 3D-printed polycaprolactone/magnesium porous scaffolds for critical size bone defect repair. ACS Biomater Sci Eng 6(9):5120–5131. https://doi.org/10.1021/acsbiomaterials.9b01911
Bobby Kannan M, Chappell J, Khakbaz H et al (2020) Biodegradable 3D porous zinc alloy scaffold for bone fracture fixation devices. Medi Dev Sens. https://doi.org/10.1002/mds3.10108
Chou D-T, Wells D, Hong D et al (2013) Novel processing of iron-manganese alloy based biomaterials by inkjet 3D printing. Acta Biomater 9(10):1016. https://doi.org/10.1016/j.actbio.2013.04.016
Martinez D, Han S, Kim N (2018) Magnesium alloy 3D printing by wire and arc additive manufacturing (WAAM). MRS Adv 3:1–6. https://doi.org/10.1557/adv.2018.553
Xu W, Zhuang Y, Zhang X et al (2019) Preparation of medical magnesium matrix composite for bone defect and design method of 3D printed material. Sci Adv Mater 11(6):824–834. https://doi.org/10.1166/sam.2019.3557
Pei X, Ma L, Zhang B et al (2017) Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication 9(4):045008. https://doi.org/10.1088/1758-5090/aa90ed
Driscoll JA, Lubbe R, Jakus AE et al (2020) 3D-printed ceramic-demineralized bone matrix hyperelastic bone composite scaffolds for spinal fusion. Tissue Eng Part A 26(3–4):157–166. https://doi.org/10.1089/ten.tea.2019.0166
Gmeiner R, Mitteramskogler G, Stampfl J et al (2015) Stereolithographic ceramic manufacturing of high strength bioactive glass. Int J Appl Ceram Technol 12(1):38–45. https://doi.org/10.1111/ijac.12325
Tesavibul P, Felzmann R, Gruber S et al (2012) Processing of 45S5 bioglass (R) by lithography-based additive manufacturing. Mater Lett 74:81–84. https://doi.org/10.1016/j.matlet.2012.01.019
Hartmann M, Pfaffinger M, Stampfl J (2021) the role of solvents in lithography-based ceramic manufacturing of lithium disilicate. Materials 14(4):1045. https://doi.org/10.3390/ma14041045
Baumgartner S, Gmeiner R, Schoenherr JA et al (2020) Stereolithography-based additive manufacturing of lithium disilicate glass ceramic for dental applications. Mater Sci Eng C-Mater Biol Appl 116:111180. https://doi.org/10.1016/j.msec.2020.111180
Li X, Yuan Y, Liu L et al (2020) 3D printing of hydroxyapatite/tricalcium phosphate scaffold with hierarchical porous structure for bone regeneration. Bio-Des Manuf 3(1):15–29. https://doi.org/10.1007/s42242-019-00056-5
Raja N, Sung A, Park H et al (2021) Low-temperature fabrication of calcium deficient hydroxyapatite bone scaffold by optimization of 3D printing conditions. Ceram Int 47(5):7005–7016. https://doi.org/10.1016/j.ceramint.2020.11.051
Mirkhalaf M, Dao A, Schindeler A et al (2021) Personalized baghdadite scaffolds: stereolithography, mechanics and in vivo testing. Acta Biomater 15(132):217–226. https://doi.org/10.1016/j.actbio.2021.03.012
Fernandes MH, Alves MM, Cebotarenco M et al (2020) Citrate zinc hydroxyapatite nanorods with enhanced cytocompatibility and osteogenesis for bone regeneration. Mater Sci Eng C-Mater Biol Appl 115:111147. https://doi.org/10.1016/j.msec.2020.111147
Koksal OK, Wrobel P, Apaydin G et al (2019) Elemental analysis for iron, cobalt, copper and zinc decorated hydroxyapatite synthetic bone dusts by EDXRF and SEM. Microchem J 144:83–87. https://doi.org/10.1016/j.microc.2018.08.050
Chen S, Shi Y, Zhang X et al (2019) Biomimetic synthesis of Mg-substituted hydroxyapatite nanocomposites and three-dimensional printing of composite scaffolds for bone regeneration. J Biomed Mater Res Part A 107(11):1549–3296. https://doi.org/10.1002/jbm.a.36757
Deng C, Yao Q, Feng C et al (2017) 3D printing of bilineage constructive biomaterials for bone and cartilage regeneration. Adv Func Mater 27:1703117. https://doi.org/10.1002/adfm.201703117
Inzana JA, Olvera D, Fuller SM et al (2014) 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 35(13):4026–4034. https://doi.org/10.1016/j.biomaterials.2014.01.064
Huang T, Fan C, Zhu M et al (2019) 3D-printed scaffolds of biomineralized hydroxyapatite nanocomposite on silk fibroin for improving bone regeneration. Appl Surf Sci 467:345–353. https://doi.org/10.1016/j.apsusc.2018.10.166
Yao Q, Wei B, Guo Y et al (2015) Design, construction and mechanical testing of digital 3D anatomical data-based PCL–HA bone tissue engineering scaffold. J Mater Sci Mater Med. https://doi.org/10.1007/s10856-014-5360-8
Hassanajili S, Karami-Pour A, Oryan A et al (2019) Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater Sci Eng C-Mater Biol Appl 104:109960. https://doi.org/10.1016/j.msec.2019.109960
Belaid H, Nagarajan S, Barou C et al (2020) Boron nitride based nanobiocomposites: design by 3d printing for bone tissue engineering. ACS Appl Bio Mater 3(4):1865–1874. https://doi.org/10.1021/acsabm.9b00965
Grottkau BE, Hui Z, Yao Y et al (2020) Rapid fabrication of anatomically-shaped bone scaffolds using indirect 3D Printing and perfusion techniques. Int J Mol Sci 21(1):315. https://doi.org/10.3390/ijms21010315
Li X, Wang Y, Wang Z et al (2018) Composite PLA/PEG/nHA/Dexamethasone scaffold prepared by 3D printing for bone regeneration. Macromol Biosci 18(6):e1800068. https://doi.org/10.1002/mabi.201800068
Bose S, Koski C, Vu AA (2020) Additive manufacturing of natural biopolymers and composites for bone tissue engineering. Mater Horiz 7(8):2011–2027. https://doi.org/10.1039/d0mh00277a
Hung BP, Naved BA, Nyberg EL et al (2016) Three-dimensional printing of bone extracellular matrix for craniofacial regeneration. ACS Biomater Sci Eng 2(10):1806–1816. https://doi.org/10.1021/acsbiomaterials.6b00101
Lee H, Yang GH, Kim M et al (2018) Fabrication of micro/nanoporous collagen/dECM/silk-fibroin biocomposite scaffolds using a low temperature 3D printing process for bone tissue regeneration. Mater Sci Eng C-Mater Biol Appl 84:140–147. https://doi.org/10.1016/j.msec.2017.11.013
Lohrasbi S, Mirzaei E, Karimizade A et al (2020) Collagen/cellulose nanofiber hydrogel scaffold: physical, mechanical and cell biocompatibility properties. Cellulose 27(2):927–940. https://doi.org/10.1007/s10570-019-02841-y
Drury J, Mooney D (2003) Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24:4337–4351. https://doi.org/10.1016/S0142-9612(03)00340-5
Xu Z, Fan C, Zhang Q et al (2021) A self-thickening and self-strengthening strategy for 3d printing high-strength and antiswelling supramolecular polymer hydrogels as meniscus substitutes. Adv Func Mater 31:2100462. https://doi.org/10.1002/adfm.202100462
Ni T, Liu M, Zhang Y et al (2020) 3D bioprinting of bone marrow mesenchymal stem cell-laden silk fibroin double network scaffolds for cartilage tissue repair. Bioconjug Chem 31(8):1938–1947. https://doi.org/10.1021/acs.bioconjchem.0c00298
Jiang P, Changyou Y, Yuxiong G et al (2019) Direct ink writing of high-strength and swelling-resistant biocompatible physical-crosslinking hydrogels. Biomater Sci 7:1805–1814. https://doi.org/10.1039/C9BM00081J
Gao F, Xu Z, Liang Q et al (2018) Direct 3D printing of high strength biohybrid gradient hydrogel scaffolds for efficient repair of osteochondral defect. Adv Func Mater 28(13):1706644. https://doi.org/10.1002/adfm.201706644
Daly R, Harrington TS, Martin GD et al (2015) Inkjet printing for pharmaceutics - a review of research and manufacturing. Int J Pharm 494(2):554–567. https://doi.org/10.1016/j.ijpharm.2015.03.017
Lin H, Zhang D, Alexander PG et al (2013) Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 34(2):331–339. https://doi.org/10.1016/j.biomaterials.2012.09.048
Dybowska-Sarapuk L, Kielbasinski K, Arazna A et al (2018) Efficient inkjet printing of graphene-based elements: influence of dispersing agent on ink viscosity. Nanomaterials 8(8):602. https://doi.org/10.3390/nano8080602
Zhong M, Zhang F, Youyi Yu et al (2018) Flexible micro-supercapacitors assembled via chemically reduced graphene oxide films assisted by a laser printer. Nanotechnology 29(43):43LT01. https://doi.org/10.1088/1361-6528/aad886
Kyle S, Jessop ZM, Al-Sabah A et al (2017) ‘Printability’ of candidate biomaterials for extrusion based 3d printing: state-of-the-art. Adv Healthc Mater 6(16):1700264. https://doi.org/10.1002/adhm.201700264
Shen Y, Tang H, Huang X et al (2020) DLP printing photocurable chitosan to build bio-constructs for tissue engineering. Carbohyd Polym 235:115970. https://doi.org/10.1016/j.carbpol.2020.115970
Zhou L, Ramezani H, Sun M et al (2020) 3D printing of high-strength chitosan hydrogel scaffolds without any organic solvents. Biomater Sci 8(18):5020–5028. https://doi.org/10.1039/d0bm00896f
Jiang P, Lin P, Yang C et al (2020) 3D printing of dual-physical cross-linking hydrogel with ultrahigh strength and toughness. Chem Mater 32(23):9983–9995. https://doi.org/10.1021/acs.chemmater.0c02941
Li Q, Xu Z, Zhang D et al (2020) T-shaped trifunctional crosslinker-toughening hydrogels. Sci China-Technol Sci 63(9):1721–1729. https://doi.org/10.1007/s11431-020-1537-6
Song X, Shi D, Song P et al (2021) Fused deposition modeling of poly(ether ether ketone) scaffolds. High Temp Mater Process (London) 40(1):1–11. https://doi.org/10.1515/htmp-2021-0009
Bertassoni LE, Cardoso JC, Manoharan V et al (2014) Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 6(2):024105. https://doi.org/10.1088/1758-5082/6/2/024105
Billiet T, Gevaert E, De Schryver T et al (2014) The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 35(1):49–62. https://doi.org/10.1016/j.biomaterials.2013.09.078
Liu W, Heinrich MA, Zhou Y et al (2017) Extrusion bioprinting of shear‐thinning gelatin methacryloyl bioinks. Adv Healthc Mater 6(12):1601451. https://doi.org/10.1002/adhm.201601451
Gasek N, Weiss DJ (2020) Effect of temperature on gelation and cross-linking of gelatin methacryloyl for biomedical applications. Phys Fluids 32(3):033102. https://doi.org/10.1063/1.5144896
Avallone PR, Raccone E, Costanzo S et al (2021) Gelation kinetics of aqueous gelatin solutions in isothermal conditions via rheological tools. Food Hydrocoll 111:106248. https://doi.org/10.1016/j.foodhyd.2020.106248
Yin J, Yan M, Wang Y et al (2018) 3d bioprinting of low-concentration cell-laden gelatin methacrylate (GelMA) bioinks with a two-step cross-linking strategy. ACS Appl Mater Interfaces 10(8):6849–6857. https://doi.org/10.1021/acsami.7b16059
Xavier JR, Thakur T, Desai P et al (2015) Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS Nano 9(3):3109–3118. https://doi.org/10.1021/nn507488s
Liu W, Zhong Z, Ning H et al (2018) Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication 10(2):024102. https://doi.org/10.1088/1758-5090/aa9d44
Gao Q, Niu X, Shao L et al (2019) 3D printing of complex GelMA-based scaffolds with nanoclay. Biofabrication 11(3):035006. https://doi.org/10.1088/1758-5090/ab0cf6
Wang Y, Huang X, Shen Y et al (2019) Direct writing alginate bioink inside pre-polymers of hydrogels to create patterned vascular networks. J Mater Sci 54:7883–7892. https://doi.org/10.1007/s10853-019-03447-2
Ansari S, Sarrion P, Hasani-Sadrabadi MM et al (2017) Regulation of the fate of dental-derived mesenchymal stem cells using engineered alginate-GelMA hydrogels. J Biomed Mater Res Part A 105(11):2957–2967. https://doi.org/10.1002/jbm.a.36148
Kesti M, Mueller M, Becher J et al (2015) A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater 11:162–172. https://doi.org/10.1016/j.actbio.2014.09.033
Duan B, Kapetanovic E, Hockaday LA et al (2014) Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater 10(5):1836–1846. https://doi.org/10.1016/j.actbio.2013.12.005
Abar B, Alonso Calleja A, Kelly A et al (2020) 3D printing of high-strength, porous, elastomeric structures to promote tissue integration of implants. J Biomed Mater Res Part A. https://doi.org/10.1002/jbm.a.37006
Zhong L, Chen J, Ma Z et al (2020) 3D printing of metal-organic framework incorporated porous scaffolds to promote osteogenic differentiation and bone regeneration. Nanoscale 12(48):24437–24449. https://doi.org/10.1039/d0nr06297a
Wang X, Fang J, Zhu W et al (2021) Bioinspired highly anisotropic, ultrastrong and stiff, and osteoconductive mineralized wood hydrogel composites for bone repair. Adv Func Mater 31:2010068. https://doi.org/10.1002/adfm.202010068
Wan Z, Zhang P, Liu Y et al (2020) Four-dimensional bioprinting: current developments and applications in bone tissue engineering. Acta Biomater 101:26–42. https://doi.org/10.1016/j.actbio.2019.10.038
Kim SH, Seo YB, Yeon YK et al (2020) 4D-bioprinted silk hydrogels for tissue engineering. Biomaterials 260:120281. https://doi.org/10.1016/j.biomaterials.2020.120281
Darabi MA, Khosrozadeh A, Wang Y et al (2020) An Alkaline based method for generating crystalline, strong, and shape memory polyvinyl alcohol biomaterials. Adv Sci 7(21):1902740. https://doi.org/10.1002/advs.201902740
Hua M, Wu D, Wu S et al (2021) 4D printable tough and thermoresponsive hydrogels. ACS Appl Mater Interfaces 13(11):12689–12697. https://doi.org/10.1021/acsami.0c17532
Senatov FS, Niaza KV, Zadorozhnyy MY et al (2016) Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J Mech Behav Biomed Mater 57:139–148. https://doi.org/10.1016/j.jmbbm.2015.11.036
Senatov FS, Zadorozhnyy MY, Niaza KV et al (2017) Shape memory effect in 3D-printed scaffolds for self-fitting implants. Eur Polymer J 93:222–231. https://doi.org/10.1016/j.eurpolymj.2017.06.011
Acknowledgements
This work has been supported by the National Natural Science Foundation of China (Grant no: 11632013, 11902214, 82103147) and Shanxi-Zheda Institute of Advanced Materials and Chemical Engineering (2021SX-AT008, 2021SX-AT009). The support of Shanxi Provincial Key Research and Development Project, China (Grant no: 201803D421060, 201803D421076) and the Natural Science Foundation of Shanxi Province, China (Grant no: 201901D111078, 201901D111077, 201801D121281) is also acknowledged with gratitude.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lan, W., Huang, X., Huang, D. et al. Progress in 3D printing for bone tissue engineering: a review. J Mater Sci 57, 12685–12709 (2022). https://doi.org/10.1007/s10853-022-07361-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07361-y