Abstract
In this article a convenient method for the synthesis of novel piperazine based bis(4-hydroxy-2H-chromen-2-one) derivatives using pyrazine-1,4-diium tricyanomethanide {[1,4-DHPyrazine][C(CN)3]2} as a new nanostructured molten salt (NMS) catalyst has been described. These compounds were synthesized via Mannich type reaction between several aromatic aldehyde, piperazine and 4-hydroxycoumarin under solvent-free condition at room temperature. The NMS catalyst was fully characterized via Fourier transform infrared (FT-IR), nuclear magnetic resonance (1H NMR and 13C NMR), mass spectrometry, thermal gravimetric, derivative thermal gravimetric, differential thermal analysis, X-ray diffraction patterns, scanning electron microscopy and transmission electron microscopy analysis. The new compounds synthesized by using this NMS catalyst were also characterized by FT-IR, 1H NMR and 13C NMR, high-resolution mass spectrometry techniques. The new NMS catalyst simply recovers and can be reused several times without significant loss of catalytic activity. The major advantages of the described method in comparison to the classical reactions are low catalyst loading, short reaction time, high yields, simple isolation of product and reusability of the NMS catalyst.
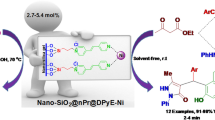
Graphical Abstract
Pyrazine-1,4-diium tricyanomethanide as a nano molten salt catalyst was designed, synthesized and used for the synthesis of novel biological piperazine based bis(4-hydroxy-2H-chromen-2- one) derivatives as bioactive and drug candidates.












Similar content being viewed by others
References
Edlund C, Oh H, Nord CE (1999) Clin Microbiol Infect 1:51–53
Chaudhary P, Kumar R, Verma AK, Singh D, Yadav V, Chhillar AK, Sharma GL, Chandra R (2006) Bioorg Med Chem 14:1819–1828
Zhao HY, Prosser AR, Liottaa DC, Wilson LJ (2015) Bioorg Med Chem Lett 25:4950–4955
Kumar A, Gupta MK, Kumar M (2011) Tetrahedron Lett 52:4521–4525
Pallavi R, Saidulu K, Javed I, Srinivas O (2012) Tetrahedron Lett 53:5314–5317
Chhanda M, Sunil R, Ray J (2012) Synth Commun 42:3077–3088
Ghosh PP, Das AR (2012) Tetrahedron Lett 53:3140–3143
Hatnapure GD, Keche AP, Rodge AH, Birajdar SS, Tale RH, Kamble VM (2012) Bioorg Med Chem Lett 22:6385–6390
Beyeh NK, Valkonen A (2010) Org Lett 12:1392–1395
Long JZ, Jin X, Adibekian A, Li WW, Cravatt BF (2010) J Med Chem 53:1830–1842
Lee YB, Gong YD, Yoon H, Ahn CH, Jeon MK, Kong JY (2010) Bioorg Med Chem 18:7966–7974
Dou D, He G, Mandadapu SR, Aravapalli S, Kim Y, Chang KO, Groutas WC (2012) Bioorg Med Chem Lett 22:377–379
Patel RV, Kumari P, Rajani DP, Pannecouque C, De Clercq E, Chikhalia KH (2012) Future Med Chem 4:1053–1065
Patel RV, Kumari P, Rajani DP (2012) J Enzyme Inhib Med Chem 27:370–374
Xu J, Cao Y, Zhang J, Yu S, Zou Y, Chai X, Wu Q, Zhang D, Jiang Y, Sun Q (2011) Eur J Med Chem 46:3142–3148
Ibezim E, Duchowicz PR, Ortiz EV, Castro EA (2012) Chemometr Intell Lab 110:81–88
Johnson KE (2007) Electrochem Soc Interface 16:38–43
Adams DJ, McDonald IR (1974) J Phys C Solid State Phys 7:2761–2773
Wilkes JS, Mamantov G, Marassi R (1987) Vol 200. D Reidel Co, Dordrecht, p 217
Wasserscheid P, Keim W (2009) Angew Chem Int Ed 39:3772–3789
Taheri A, Lai B, Cheng C, Gu Y (2015) Green Chem 17:812–816
Taheri A, Liu C, Lai B, Cheng C, Pan X, Gu Y (2014) Green Chem 16:3715–3719
Taheri A, Pan X, Liu C, Gu Y (2014) ChemSusChem 7:2094–2100
García-Verdugo E, Altava B, Burguete MI, Lozano P, Luis SV (2015) Green Chem 17:2693–2713
Luska KL, Migowski P, Leitner W (2015) Green Chem 17:3195–3206
Zhu S, Wu Y, Chen Q, Yu Z, Wang C, Jin S, Ding Y, Wu G (2006) Green Chem 8:325–327
Van Rantwijk F, Lau RM, Sheldon RA (2003) Trends Biotechnol 21:131–138
Dupont J, Fonseca GS, Umpierre AP, Fichtner PFP, Teixeira SR (2002) J Am Chem Soc 124:4228–4229
Reichardt C (2007) Org Process Res 11:105–113
Sundermeyer W (1965) Angew Chem Int Ed Engl 4:222–238
Parvulescu VI, Hardacre C (2007) Chem Rev 107:2615–2665
Welton T (1999) Chem Rev 99:2071–2083
Lei Z, Dai C, Chen B (2014) Chem Rev 114:1289–1326
Hayes R, Gregory G, Warr GG, Atkin R (2015) Chem Rev 115:6357–6426
Amarasekara AS (2016) Chem Rev 116:6133–6183
Egorova KS, Ananikov VP (2014) ChemSusChem 7:336–360
Frade RFM, Afonso CAM (2010) Hum Exp Toxicol 29:1038–1054
Jastorff B, Störmann R, Ranke J, Mölter K, Stock F, Oberheitmann B, Hoffmann W, Hoffmann J, Nüchter M, Ondruschka B, Filser J (2003) Green Chem 5:136–142
Kianpour E, Azizian S, Yarie M, Zolfigol MA, Bayat M (2016) Chem Eng J 295:500–508
Zolfigol MA, Yarie M, Baghery S (2016) Synlett 27:1418–1422
Zolfigol MA, Mansouri N, Baghery S (2016) Synlett 27:1511–1515
Ghaderi H, Zolfigol MA, Bayat Y, Zarei M, Noroozizadeh E (2016) Synlett 27:2246–2250
Zolfigol MA, Khazaei A, Moosavi-Zare AR, Zare A, Kruger HG, Asgari Z, Khakyzadeh V, Kazem-Rostami M (2012) J Org Chem 77:3640–3645
Moosavi-Zare AR, Zolfigol MA, Zarei M, Noroozizadeh E, Beyzavi MH (2016) RSC Adv 6:89572–89577
Moosavi-Zare AR, Zolfigol MA, Noroozizadeh E (2016) Synlett 27:1682–1684
Zolfigol MA, Afsharnadery F, Baghery S, Salehzadeh S, Maleki F (2015) RSC Adv 5:75555–75568
Zolfigol MA, Khazaei A, Alaie S, Baghery S, Maleki F, Bayat Y, Asghari A (2016) RSC Adv 6:58667–58679
Zolfigol MA, Kiafar M, Yarie M, Taherpour A, Saeidirad M (2016) RSC Adv 6:50100–50111
Verkade JMM, Van Hemert LJC, Quaedflieg PJLM, Rutjes FPJT (2008) Chem Soc Rev 37:29–41
Hayashi Y, Urushima T, Shin M, Shoji M (2005) Tetrahedron 61:11393–11404
Zolfigol MA, Bahrami-Nejad N, Afsharnadery F, Baghery S (2016) J Mol Liq 221:851–859
Safaiee M, Zolfigol MA, Bahrami-Nejad N, Afsharnadery F, Baghery S (2015) RSC Adv 5:102340–102349
Yu B, Xie JN, Zhong CL, Li W, He LN (2015) ACS Catal 8:3940–3944
Acknowledgements
We thank Bu-Ali Sina University and Iran National Science Foundation (INSF) for financial support (Grant of Allameh Tabataba’i’s Award, Grant Number BN093), and National Elites Foundation to our research groups.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baghery, S., Zolfigol, M.A., Schirhagl, R. et al. {[1,4-DHPyrazine][C(CN)3]2} as a New Nano Molten Salt Catalyst for the Synthesis of Novel Piperazine Based bis(4-hydroxy-2H-chromen-2-one) Derivatives. Catal Lett 147, 2083–2099 (2017). https://doi.org/10.1007/s10562-017-2096-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2096-3