Abstract
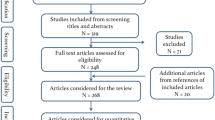
The transition to performing procedures robotically generally entails a period of adjustment known as a learning curve as the surgeon develops a familiarity with the technology. However, no study has comprehensively examined robotic learning curves across the field of neurosurgery. We conducted a systematic review to characterize the scope of literature on robotic learning curves in neurosurgery, assess operative parameters that may involve a learning curve, and delineate areas for future investigation. PubMed, Embase, and Scopus were searched. Following deduplication, articles were screened by title and abstract for relevance. Remaining articles were screened via full text for final inclusion. Bibliographic and learning curve data were extracted. Of 746 resultant articles, 32 articles describing 3074 patients were included, of which 23 (71.9%) examined spine, 4 (12.5%) pediatric, 4 (12.5%) functional, and 1 (3.1%) general neurosurgery. The parameters assessed for learning curves were heterogeneous. In total, 8 (57.1%) of 14 studies found reduced operative time with increased cases, while the remainder demonstrated no learning curve. Six (60.0%) of 10 studies reported reduced operative time per component with increased cases, while the remainder indicated no learning curve. Radiation time, radiation time per component, robot time, registration time, setup time, and radiation dose were assessed by ≤ 4 studies each, with 0–66.7% of studies demonstrated a learning curve. Four (44.4%) of 9 studies on accuracy showed improvement over time, while the others indicated no improvement over time. The number of cases required to reverse the learning curve ranged from 3 to 75. Learning curves are common in robotic neurosurgery. However, existing studies demonstrate high heterogeneity in assessed parameters and the number of cases that comprise the learning curve. Future studies should seek to develop strategies to reduce the number of cases required to reach the learning curve.


Similar content being viewed by others
Data availability
All relevant data are provided in the manuscript.
References
Mattei TA, Rodriguez AH, Sambhara D, Mendel E (2014) Current state-of-the-art and future perspectives of robotic technology in neurosurgery. Neurosurg Rev 37(3):357–366
Howe RD, Matsuoka Y (1999) Robotics for surgery. Annu Rev Biomed Eng 1(1):211–240
Kwoh YS, Hou J, Jonckheere EA, Hayati S (1988) A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng 35(2):153–160
Khanna O, Beasley R, Franco D, DiMaio S (2021) The path to surgical robotics in neurosurgery. Operative Neurosurgery 20(6):514–520
Ball T, González-Martínez J, Zemmar A et al (2021) Robotic applications in cranial neurosurgery: current and future. Operative Neurosurgery 21(6):371–379
Pennington Z, Judy BF, Zakaria HM et al (2022) Learning curves in robot-assisted spine surgery: a systematic review and proposal of application to residency curricula. Neurosurg Focus 52(1):E3
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906
Guyatt GH, Oxman AD, Vist GE et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926
Sterne JA, Hernán MA, Reeves BC, et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. bmj.;355
Abbas R, Al Saiegh F, El Naamani K et al (2022) Robot-assisted carotid artery stenting: outcomes, safety, and operational learning curve. Neurosurg Focus 52(1):E17
Avrumova F, Morse KW, Heath M, Widmann RF, Lebl DR (2021) Evaluation of K-wireless robotic and navigation assisted pedicle screw placement in adult degenerative spinal surgery: learning curve and technical notes. Journal of Spine Surgery 7(2):141
Bäcker HC, Freibott CE, Perka C, Weidenbaum M (2020) Surgeons’ learning curve of Renaissance robotic surgical system. International Journal of Spine Surgery 14(5):818–823
Barzilay Y, Liebergall M, Fridlander A, Knoller N (2006) Miniature robotic guidance for spine surgery—introduction of a novel system and analysis of challenges encountered during the clinical development phase at two spine centres. Int J Med Rob Comp Assist Surg 2(2):146–153
Bydon M, Chen SG, Neal MD, et al (2021) Initiation of a robotic program in spinal surgery: experience at a three-site medical center. Elsevier 1193–1202.
Chang M, Wang L, Yuan S, Tian Y (2022) Percutaneous endoscopic robot-assisted transforaminal lumbar interbody fusion (PE RA-TLIF) for lumbar spondylolisthesis: a technical note and two years clinical results. Pain Physician 25:E73–E86
Chen X, Song Q, Wang K et al (2021) Robot-assisted minimally invasive transforaminal lumbar interbody fusion versus open transforaminal lumbar interbody fusion: a retrospective matched-control analysis for clinical and quality-of-life outcomes. J Comp Effect Res 10(10):845–856
Chesney K, Triano M, Dowlati E, Zhang I, Felbaum DR, Aulisi EF (2021) Cirq robotic arm-assisted transpedicular instrumentation with intraoperative navigation: technical note and case series with 714 thoracolumbar screws. Journal of robotic surgery 1–6
Gy Cui, Xg Han, Wei Y et al (2021) Robot-assisted minimally invasive transforaminal lumbar interbody fusion in the treatment of lumbar spondylolisthesis. Orthopaedic Surgery 13(7):1960–1968
De Biase G, Gassie K, Garcia D et al (2021) Perioperative comparison of robotic-assisted versus fluoroscopically guided minimally invasive transforaminal lumbar interbody fusion. World Neurosurgery 149:e570–e575
Dlaka D, Švaco M, Chudy D et al (2021) Frameless stereotactic brain biopsy: a prospective study on robot-assisted brain biopsies performed on 32 patients by using the RONNA G4 system. Int J Med Rob Comp Assist Surg 17(3):e2245
Fayed I, Tai A, Triano M et al (2020) Robot-assisted percutaneous pedicle screw placement: evaluation of accuracy of the first 100 screws and comparison with cohort of fluoroscopy-guided screws. World neurosurgery 143:e492–e502
Furlanetti L, Ellenbogen J, Gimeno H et al (2021) Targeting accuracy of robot-assisted deep brain stimulation surgery in childhood-onset dystonia: a single-center prospective cohort analysis of 45 consecutive cases. J Neurosurg Pediatr 27(6):677–687
Gonen L, Chakravarthi SS, Monroy-Sosa A et al (2017) Initial experience with a robotically operated video optical telescopic-microscope in cranial neurosurgery: feasibility, safety, and clinical applications. Neurosurg Focus 42(5):E9
Ho AL, Muftuoglu Y, Pendharkar AV et al (2018) Robot-guided pediatric stereoelectroencephalography: single-institution experience. J Neurosurg Pediatr 22(5):489–496
Hoshide R, Calayag M, Meltzer H, Levy ML, Gonda D (2017) Robot-assisted endoscopic third ventriculostomy: institutional experience in 9 patients. J Neurosurg Pediatr 20(2):125–133
Hu X, Lieberman IH (2014) What is the learning curve for robotic-assisted pedicle screw placement in spine surgery? Clinical Orthopaedics and Related Research® 472(6):1839–1844
Hyun S-J, Kim K-J, Jahng T-A, Kim H-J (2017) Minimally invasive robotic versus open fluoroscopic-guided spinal instrumented fusions: a randomized controlled trial. Spine 42(6):353–358
Jiang B, Pennington Z, Azad T et al (2020) Robot-assisted versus freehand instrumentation in short-segment lumbar fusion: experience with real-time image-guided spinal robot. World neurosurgery 136:e635–e645
Kam JK, Gan C, Dimou S et al (2019) Learning curve for robot-assisted percutaneous pedicle screw placement in thoracolumbar surgery. Asian Spine Journal 13(6):920
Khan A, Meyers JE, Siasios I, Pollina J (2019) Next-generation robotic spine surgery: first report on feasibility, safety, and learning curve. Operative Neurosurgery 17(1):61–69
Khan A, Rho K, Mao JZ et al (2020) Comparing cortical bone trajectories for pedicle screw insertion using robotic guidance and three-dimensional computed tomography navigation. World Neurosurgery 141:e625–e632
Kim HJ, Jung WI, Chang BS, Lee CK, Kang KT, Yeom JS (2017) A prospective, randomized, controlled trial of robot-assisted vs freehand pedicle screw fixation in spine surgery. Int J Med Rob Comp Assist Surg 13(3):e1779
Kim LH, Feng AY, Ho AL et al (2020) Robot-assisted versus manual navigated stereoelectroencephalography in adult medically-refractory epilepsy patients. Epilepsy Res 159:106253
Machetanz K, Grimm F, Schuhmann M, Tatagiba M, Gharabaghi A, Naros G (2021) Time efficiency in stereotactic robot-assisted surgery: an appraisal of the surgical procedure and surgeon’s learning curve. Stereotact Funct Neurosurg 99(1):25–33
Mallereau C-H, Chibbaro S, Ganau M et al (2022) Pushing the boundaries of accuracy and reliability during stereotactic procedures: a prospective study on 526 biopsies comparing the frameless robotic and Image-Guided Surgery systems. J Clin Neurosci 95:203–212
McGovern RA, Butler RS, Bena J, Gonzalez-Martinez J (2020) Incorporating new technology into a surgical technique: the learning curve of a single surgeon’s stereo-electroencephalography experience. Neurosurgery 86(3):E281–E289
Morse KW, Heath M, Avrumova F et al (2021) Comprehensive error analysis for robotic-assisted placement of pedicle screws in pediatric spinal deformity: the initial learning curve. J Pediat Orthop 41(7):e524–e532
Onen MR, Simsek M, Nader S (2014) Robotic spine surgery: a preliminary report. Turk Neurosurg 24(4):512–518
Schatlo B, Martinez R, Alaid A et al (2015) Unskilled unawareness and the learning curve in robotic spine surgery. Acta Neurochir 157(10):1819–1823
Shi B, Hu L, Du H, Zhang J, Zhao W, Zhang L (2021) Robot-assisted percutaneous vertebroplasty under local anaesthesia for osteoporotic vertebral compression fractures: a retrospective, clinical, non-randomized, controlled study. Int J Med Rob Comp Assist Surg 17(3):e2216
Siddiqui MI, Wallace DJ, Salazar LM, Vardiman AB (2019) Robot-assisted pedicle screw placement: learning curve experience. World Neurosurgery 130:e417–e422
Urakov TM, Chang KH-k, Burks SS, Wang MY (2017) Initial academic experience and learning curve with robotic spine instrumentation. Neurosurgical focus 42(5):E4
Vakharia VN, Rodionov R, Miserocchi A et al (2021) Comparison of robotic and manual implantation of intracerebral electrodes: a single-centre, single-blinded, randomised controlled trial. Sci Rep 11(1):1–10
van Dijk JD, van den Ende RP, Stramigioli S, Köchling M, Höss N (2015) Clinical pedicle screw accuracy and deviation from planning in robot-guided spine surgery: robot-guided pedicle screw accuracy. Spine 40(17):E986–E991
Wang TY, Mehta VA, Sankey EW, Lavoie S, Abd-El-Barr MM, Yarbrough CK (2021) Operative time and learning curve between fluoroscopy-based instrument tracking and robot-assisted instrumentation for patients undergoing minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF). Clin Neurol Neurosurg 206:106698
Wood MJ, McMillen J (2014) The surgical learning curve and accuracy of minimally invasive lumbar pedicle screw placement using CT based computer-assisted navigation plus continuous electromyography monitoring–a retrospective review of 627 screws in 150 patients. International journal of spine surgery 8
Yu J, Zhang Q, Fan M-X, Han X-G, Liu B, Tian W (2021) Learning curves of robot-assisted pedicle screw fixations based on the cumulative sum test. World Journal of Clinical Cases 9(33):10134
Yuan W, Cao W, Meng X et al (2020) Learning curve of robot-assisted percutaneous kyphoplasty for osteoporotic vertebral compression fractures. World neurosurgery 138:e323–e329
Marcus HJ, Vakharia VN, Ourselin S, Duncan J, Tisdall M, Aquilina K (2018) Robot-assisted stereotactic brain biopsy: systematic review and bibliometric analysis. Childs Nerv Syst 34(7):1299–1309
Varma T, Eldridge P (2006) Use of the NeuroMate stereotactic robot in a frameless mode for functional neurosurgery. Int J Med Rob Comp Assist Surg 2(2):107–113
von Langsdorff D, Paquis P, Fontaine D (2015) In vivo measurement of the frame-based application accuracy of the Neuromate neurosurgical robot. J Neurosurg 122(1):191–194
Cardinale F, Cossu M, Castana L et al (2013) Stereoelectroencephalography: surgical methodology, safety, and stereotactic application accuracy in 500 procedures. Neurosurgery 72(3):353–366
Philipp LR, Matias CM, Thalheimer S, Mehta SH, Sharan A, Wu C (2021) Robot-assisted stereotaxy reduces target error: a meta-analysis and meta-regression of 6056 trajectories. Neurosurgery 88(2):222–233
Fomenko A, Serletis D (2018) Robotic stereotaxy in cranial neurosurgery: a qualitative systematic review. Neurosurgery 83(4):642–650
Menaker SA, Shah SS, Snelling BM, Sur S, Starke RM, Peterson EC (2018) Current applications and future perspectives of robotics in cerebrovascular and endovascular neurosurgery. J Neurointerv Surg 10(1):78–82
Smith AD, Teague AJ, Naik A et al (2022) Robotic external ventricular drain placement for acute neurosurgical care in low-resource settings: feasibility considerations and a prototype design. Neurosurg Focus 52(1):E14
Xiong R, Li F, Chen X (2020) Robot-assisted neurosurgery versus conventional treatment for intracerebral hemorrhage: a systematic review and meta-analysis. J Clin Neurosci 82:252–259
Wagner CR, Phillips T, Roux S, Corrigan JP (2021) Future directions in robotic neurosurgery. Operative Neurosurgery 21(4):173–180
Vilanilam GC, Venkat EH (2022) Ethical nuances and medicolegal vulnerabilities in robotic neurosurgery. Neurosurg Focus 52(1):E2
Bareeq RA, Jayaraman S, Kiaii B, Schlachta C, Denstedt JD, Pautler SE (2008) The role of surgical simulation and the learning curve in robot-assisted surgery. J Robot Surg 2(1):11–15
Gao S, Lv Z, Fang H (2018) Robot-assisted and conventional freehand pedicle screw placement: a systematic review and meta-analysis of randomized controlled trials. Eur Spine J 27(4):921–930
Naik A, Smith AD, Shaffer A et al (2022) Evaluating robotic pedicle screw placement against conventional modalities: a systematic review and network meta-analysis. Neurosurg Focus 52(1):E10
Kaul S, Shah NL, Menon M (2006) Learning curve using robotic surgery. Curr Urol Rep 7(2):125–129
Author information
Authors and Affiliations
Contributions
NAS conceptualized the idea, conducted the screening and data extraction, prepared figures and tables, and wrote the manuscript draft. JH conducted the screening and data extraction and assisted in revising the manuscript. CW conceptualized the idea, assisted in revising the manuscript, and provided project supervision. All authors reviewed and approved the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors have approved the final version of this manuscript.
Conflict of interest
The authors declare no competing interests.
Human and animal ethics
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
No part of this work has been previously published.
Supplementary Information
ESM 1
(DOCX 13.8 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shlobin, N.A., Huang, J. & Wu, C. Learning curves in robotic neurosurgery: a systematic review. Neurosurg Rev 46, 14 (2023). https://doi.org/10.1007/s10143-022-01908-y
Published:
DOI: https://doi.org/10.1007/s10143-022-01908-y