Abstract
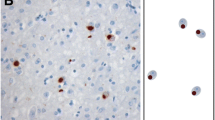
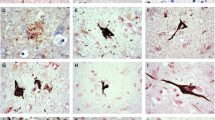
Amyotrophic lateral sclerosis with dementia (ALSD), corresponding to the motor neuron disease type of frontotemporal dementia, is neuropathologically characterized by depletion of the motor neurons, degeneration of the extra-motor cerebral cortices and formation of ubiquitin-immunoreactive (not argyrophilic, tau-negative, α-synuclein-negative) intraneuronal inclusions. Recently, immunoreactivity for ubiquitin-binding protein p62 has been reported in several ubiquitin-containing intraneuronal or intraglial inclusions (e.g. neurofibrillary tangles, Pick bodies, Lewy bodies, glial cytoplasmic inclusions) in various neurodegenerative diseases. We examined p62 immunoreactivity in ubiquitin-immunoreactive intraneuronal inclusions in five ALSD cases with a broad clinicopathological spectrum. p62 immunoreactivity in ubiquitin-immunoreactive intraneuronal inclusions was seen in all cases. The mean proportion of p62-immunoreactive inclusions to the total number of ubiquitin-immunoreactive inclusions (p62/Ub ratio) in the dentate gyrus was 27.5±16.6% (range 6.3–47.3%). There was no correlation between p62/Ub ratio and the severity of dementia, duration of illness or neuropathological severity. Although the main constituent of these inclusions is unknown, our study suggests that p62 contributes to the formation of the inclusions via the same mechanism as in other previously reported neurodegenerative diseases. Since p62 is believed to have a neuroprotective role, the formation of these inclusions may represent a non-harmful, rather protective effect against the neuronal degeneration in ALSD.


Similar content being viewed by others
References
Anderson VER, Cairns NJ, Leigh PN (1995) Involvement of the amygdala, dentate and hippocampus in motor neuron disease. J Neurol Sci 129:75–78
Chang L, Cornford M, Miller BL, Itabashi H, Mena I (1995) Neuronal ultrastructural abnormalities in a patient with frontotemporal dementia and motor neuron disease. Dementia 6:1–8
Joung I, Strominger JL, Shin J (1996) Molecular cloning of a phosphotyrosine-independent ligand of the p56lck SH2 domain. Proc Natl Acad Sci USA 93:5991–5995
Kato S, Oda M, Hayashi H (1993) Neuropathology in amyotrophic lateral sclerosis patients on respirators: uniformity and diversity in 13 cases. Neuropathology 13:229–236
Kato S, Oda M, Hayashi H, Kawata A, Shimizu T (1994) Participation of the limbic system and its associated areas in the dementia of amyotrophic lateral sclerosis. J Neurol Sci 126:62–69
Komachi H, Okeda R, Ishii N, Yanagisawa K, Yamada M, Miyatake T (1994) Motor neuron disease with dementia and ophthalmoplegia. A clinicopathological study. J Neurol 241:592–596
Kuusisto E, Salminen A, Alafuzoff I (2001) Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. Neuroreport 12:2085–2090
Kuusisto E, Suuronen T, Salminen A (2001) Ubiquitin-binding protein p62 expression is induced during apoptosis and proteasomal inhibition in neuronal cells. Biochem Biophys Res Commun 280:223–228
Kuusisto E, Salminen A, Alafuzoff I (2002) Early accumulation of p62 in neurofibrillary tangles in Alzheimer’s disease: possible role in tangle formation. Neuropathol Appl Neurobiol 28:228–237
Mitsuyama Y (1984) Presenile dementia with motor neuron disease in Japan: clinico-pathological review of 26 cases. J Neurol Neurosurg Psychiatry 47:953–959
Mitsuyama Y (1993) Presenile dementia with motor neuron disease. Dementia 4:137–142
Mizutani T, Sakamaki S, Tsuchiya N, Kamei S, Kohzu H, Horiuchi R, Ida M, Shiozawa R, Takasu T (1992) Amyotrophic lateral sclerosis with ophthalmoplegia and multisystem degeneration in patients on long-term use of respirators. Acta Neuropathol 84:372–377
Nakano I (1993) Temporal lobe lesions in amyotrophic lateral sclerosis with or without dementia: a neuropathological study. Neuropathology 13:215–227
Nakano I, Iwatsubo T, Hashizume Y, Mizutani T, Mannen T (1992) Amyotrophic lateral sclerosis with dementia. Lesions in the apical cortex and some deeper structures of temporal lobes. Neuropathology 12:69–77
Neary D, Snowden JS, Mann DMA, Northen B, Goulding PJ, Macdermott N (1990) Frontotemporal lobe dementia and motor neuron disease. J Neurol Neurosurg Psychiatry 53:23–32
Okamoto K, Hirai S, Yamazaki T, Sun X, Nakazato Y (1991) New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett 129:233–236
Park I, Chung J, Walsh CT, Yun Y, Strominger JL, Shin J (1995) Phosphotyrosine-independent binding of a 62-kDa protein to the src homology 2 (SH2) domain of p56lck and its regulation by phosphorylation of Ser-59 in the lck unique N-terminal region. Proc Natl Acad Sci USA 92:12338–12342
Samuels IS, Seibenhener ML, Neidigh KBW, Wooten MW (2001) Nerve growth factor stimulates the interaction of ZIP/p62 with atypical protein kinase C and targets endosomal localization: evidence for regulation of nerve growth factor-induced differentiation. J Cell Biochem 82:452–466
Sasaki S, Tsutsumi Y, Yamane K, Sakuma H, Maruyama S (1992) Sporadic amyotrophic lateral sclerosis with extensive neurological involvement. Acta Neuropathol 84:211–215
Shin J (1998) P62 and the sequestosome, a novel mechanism for protein metabolism. Arch Pharm Res 21:629–633
Takeda S, Yamada M, Kawasaki K, Oyanagi K, Ikuta F, Arai M, Inuzuka T, Yuki N, Yuasa T, Sato S, Tsuji S, Miyatake T (1994) Motor neuron disease with multi-system involvement presenting as tetraparesis, ophthalmoplegia and sensori-autonomic dysfunction. Acta Neuropathol 88:193–200
The Lund and Manchester Groups (1994) Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry 57:416–418
Vadlamudi RK, Joung I, Strominger JL, Shin J (1996) p62, a phosphotyrosine-independent ligand of the SH2 domain of p56lck, belongs to a new class of ubiquitin-binding proteins. J Biol Chem 271:20235–20237
Wakabayashi K, Piao YS, Hayashi S, Kakita A, Yamada M, Takahashi H (2001) Ubiquitinated neuronal inclusions in the neostriatum in patients with amyotrophic lateral sclerosis with and without dementia. A study of 60 patients 31 to 87 years of age. Clin Neuropathol 20:47–52
Wightman G, Anderson VER, Martin J, Swash M, Anderton BH, Neary D, Mann D, Luthert P, Leigh PN (1992) Hippocampal and neocortical ubiquitin-immunoreactive inclusions in amyotrophic lateral sclerosis with dementia. Neurosci Lett 139:269–274
Yoshida M, Murakami N, Hashizume Y, Takahashi A (1992) A clinicopathological study on 13 cases of motor neuron disease with dementia. Rinsho Shinkeigaku 32:1193–1202
Zatloukal K, Stumptner C, Fuchsbichler A, Heid H, Schnoelzer M, Kenner L, Kleinert R, Prinz M, Aguzzi A, Denk H (2002) p62 is a common component of cytoplasmic inclusions in protein aggregation diseases. Am J Pathol 160:255–263
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakano, T., Nakaso, K., Nakashima, K. et al. Expression of ubiquitin-binding protein p62 in ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis with dementia: analysis of five autopsy cases with broad clinicopathological spectrum. Acta Neuropathol 107, 359–364 (2004). https://doi.org/10.1007/s00401-004-0821-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0821-7