Abstract
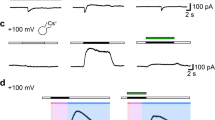
The swelling-activated chloride current (I Cl,Vol) is abundantly expressed in glioblastoma (GBM) cells, where it controls cell volume and invasive migration. The transduction pathway mediating I Cl,Vol activation in GBM cells is, however, poorly understood. By means of pharmacological and electrophysiological approaches, on GL-15 human GBM cells we found that I Cl,Vol activation by hypotonic swelling required the activity of a U73122-sensitive phospholipase C (PLC). I Cl,Vol activation could also be induced by the membrane-permeable diacylglycerol (DAG) analog OAG. In contrast, neither calcium (Ca2+) chelation by BAPTA-AM nor changes in PKC activity were able to affect I Cl,Vol activation by hypotonic swelling. We further found that R59022, an inhibitor of diacylglycerol kinase (DGK), reverted I Cl,Vol activation, suggesting the involvement of phosphatidic acid. In addition, I Cl,Vol activation required the activity of a EHT1864-sensitive Rac1 small GTPase and the resulting actin polymerization, as I Cl,Vol activation was prevented by cytochalasin B. We finally show that I Cl,Vol can be activated by the promigratory fetal calf serum in a PLC- and DGK-dependent manner. This observation is potentially relevant because blood serum can likely come in contact with glioblastoma cells in vivo as a result of the tumor-related partial breakdown of the blood–brain barrier. Given the relevance of I Cl,Vol in GBM cell volume regulation and invasiveness, the several key signaling molecules found in this study to be involved in the activation of the I Cl,Vol may represent potential therapeutic targets against this lethal cancer.





Similar content being viewed by others
References
Abramovici H, Mojtabaie P, Parks RJ, Zhong XP, Koretzky GA, Topham MK, Gee SH (2009) Diacylglycerol kinase zeta regulates actin cytoskeleton reorganization through dissociation of Rac1 from RhoGDI. Mol Biol Cell 20:2049–2059
Akita T, Fedorovich SV, Okada Y (2011) Ca2+ nanodomain-mediated component of swelling-induced volume-sensitive outwardly rectifying anion current triggered by autocrine action of ATP in mouse astrocytes. Cell Physiol Biochem 28:1181–1190
Arreola J, Begenisich T, Nehrke K, Nguyen HV, Park K, Richardson L, Yang B, Schutte BC, Lamb FS, Melvin JE (2002) Secretion and cell volume regulation by salivary acinar cells from mice lacking expression of the Clcn3 Cl− channel gene. J Physiol 545:207–216
Barfod ET, Moore AL, Melnick RF, Lidofsky SD (2005) Src regulates distinct pathways for cell volume control through Vav and phospholipase Cgamma. J Biol Chem 280:25548–25557
Bordey A, Sontheimer H, Trouslard J (2000) Muscarinic activation of BK channels induces membrane oscillations in glioma cells and leads to inhibition of cellmigration. J Membr Biol 176:31–40
Browe DM, Baumgarten CM (2006) EGFR kinase regulates volume-sensitive chloride current elicited by integrin stretch via PI-3K and NADPH oxidase in ventricular myocytes. J Gen Physiol 127:237–251
Catacuzzeno L, Aiello F, Fioretti B, Sforna L, Castigli E, Ruggieri P, Tata AM, Calogero A, Franciolini F (2011) Serum-activated K and Cl currents underlay U87-MG glioblastoma cell migration. J Cell Physiol 226:1926–1933
Catacuzzeno L, Fioretti B, Franciolini F (2012) Expression and role of the intermediate-conductance calcium-activated potassium channel KCa3.1 in glioblastoma. J Signal Transduct 2012:421564
Chianale F, Cutrupi S, Rainero E, Baldanzi G, Porporato PE, Traini S, Filigheddu N, Gnocchi VF, Santoro MM, Parolini O, van Blitterswijk WJ, Sinigaglia F, Graziani A (2007) Diacylglycerol kinase-alpha mediates hepatocyte growth factor–induced epithelial cell scatter by regulating Rac activation and membrane ruffling. Mol Biol Cell 18:4859–4871
Chianale F, Rainero E, Cianflone C, Bettio V, Pighini A, Porporato PE, Filigheddu N, Serini G, Sinigaglia F, Baldanzi G, Graziani A (2010) Diacylglycerol kinase alpha mediates HGF-induced Rac activation and membrane ruffling by regulating atypical PKC and RhoGDI. Proc Natl Acad Sci USA 107:4182–4187
Coca-Prados M, Anguíta J, Chalfant ML, Civan MM (1995) PKC-sensitive Cl− channels associated with ciliary epithelial homologue of pICln. Am J Physiol 268:C572–C579
Cornet M, Ubl J, Kolb HA (1993) Cytoskeleton and ion movements during volume regulation in cultured PC12 cells. J Membr Biol 133:161–170
Cuddapah VA, Sontheimer H (2010) Molecular interaction and functional regulation of ClC-3 by Ca2+/calmodulin-dependent protein kinase II (CaMKII) in human malignant glioma. J Biol Chem 285:11188–11196
Cuddapah VA, Sontheimer H (2011) Ion channels and transporters in cancer. 2. Ion channels and the control of cancer cell migration. Am J Physiol Cell Physiol 301:C541–C549
Deng W, Baki L, Baumgarten CM (2010) Endothelin signaling regulates volume-sensitive Cl− current via NADPH oxidase and mitochondrial reactive oxygen species. Cardiovasc Res 88:93–100
Doroshenko P, Penner R, Neher E (1991) Novel chloride conductance in the membrane of bovine chromaffin cells activated by intracellular GTP gamma S. J Physiol 436:711–724
Duan D, Fermini B, Nattel S (1995) Alpha-adrenergic control of volume-regulated Cl− currents in rabbit atrial myocytes. Characterization of a novel ionic regulatory mechanism. Circ Res 77:379–393
Duan D, Winter C, Cowley S, Hume JR, Horowitz B (1997) Molecular identification of a volume-regulated chloride channel. Nature 390:417–421
Duan D, Cowley S, Horowitz B, Hume JR (1999) A serine residue in ClC-3 links phosphorylation–dephosphorylation to chloride channel regulation by cell volume. J Gen Physiol 113:57–70
Duan D, Zhong J, Hermoso M, Satterwhite CM, Rossow CF, Hatton WJ, Yamboliev I, Horowitz B, Hume JR (2001) Functional inhibition of native volume-sensitive outwardly rectifying anion channels in muscle cells and Xenopus oocytes by anti-ClC-3 antibody. J Physiol 531:437–444
Ellershaw DC, Greenwood IA, Large WA (2002) Modulation of volume-sensitive chloride current by noradrenaline in rabbit portal vein myocytes. J Physiol 542:537–547
Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD (2002) Inhibition of gap junction hemichannels by chloride channel blockers. J Membr Biol 185:93–102
Fioretti B, Castigli E, Calzuola I, Harper AA, Franciolini F, Catacuzzeno L (2004) NPPB block of the intermediate-conductance Ca2+-activated K+ channel. Eur J Pharmacol 497:1–6
Fioretti B, Castigli E, Micheli MR, Bova R, Sciaccaluga M, Harper A, Franciolini F, Catacuzzeno L (2006) Expression and modulation of the intermediate-conductance Ca2+-activated K+ channel in glioblastoma GL-15 cells. Cell Physiol Biochem 18:47–56
Fioretti B, Catacuzzeno L, Sforna L, Aiello F, Pagani F, Ragozzino D, Castigli E, Franciolini F (2009) Histamine hyperpolarizes human glioblastoma cells by activating the intermediate-conductance Ca2+-activated K+ channel. Am J Physiol Cell Physiol 297:C102–C110
Gong W, Xu H, Shimizu T, Morishima S, Tanabe S, Tachibe T, Uchida S, Sasaki S, Okada Y (2004) ClC-3-independent, PKC-dependent activity of volume-sensitive Cl channel in mouse ventricular cardiomyocytes. Cell Physiol Biochem 14:213–224
Gosling M, Smith JW, Poyner DR (1995) Characterization of a volume-sensitive chloride current in rat osteoblast-like (ROS 17/2.8) cells. J Physiol 485:671–682
Habela CW, Ernest NJ, Swindall AF, Sontheimer H (2009) Chloride accumulation drives volume dynamics underlying cell proliferation and migration. J Neurophysiol 101:750–757
Hall A (1994) Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol 10:31–54
Hardy SP, Goodfellow HR, Valverde MA, Gill DR, Sepúlveda V, Higgins CF (1995) Protein kinase C-mediated phosphorylation of the human multidrug resistance P-glycoprotein regulates cell volume-activated chloride channels. EMBO J 14:68–75
Hermoso M, Satterwhite CM, Andrade YN, Hidalgo J, Wilson SM, Horowitz B, Hume JR (2002) ClC-3 is a fundamental molecular component of volume-sensitive outwardly rectifying Cl− channels and volume regulation in HeLa cells and Xenopus laevis oocytes. J Biol Chem 277:40066–40074
Hoffmann EK, Lambert IH, Pedersen SF (2009) Physiology of cell volume regulation in vertebrates. Physiol Rev 89:193–277
Jin NG, Kim JK, Yang DK, Cho SJ, Kim JM, Koh EJ, Jung HC, So I, Kim KW (2003) Fundamental role of ClC-3 in volume-sensitive Cl− channel function and cell volume regulation in AGS cells. Am J Physiol Gastrointest Liver Physiol 285:G938–G948
Lui VC, Lung SS, Pu JK, Hung KN, Leung GK (2010) Invasion of human glioma cells is regulated by multiple chloride channels including ClC-3. Anticancer Res 30:4515–4524
Lund CV, Nguyen MT, Owens GC, Pakchoian AJ, Shaterian A, Kruse CA, Eliceiri BP (2006) Reduced glioma infiltration in Src-deficient mice. J Neurooncol 78:19–29
Manning TJ Jr, Parker JC, Sontheimer H (2000) Role of lysophosphatidic acid and rho in glioma cell motility. Cell Motil Cytoskelet 45:185–199
Mariggio MA, Mazzoleni G, Pietrangelo T, Guarnieri S, Morabito C, Steimberg N, Fano G (2001) Calcium-mediated transductive systems and functionally active gap junctions in astrocyte-like GL15 cells. BMC Physiol 1:4
McCloskey DT, Doherty L, Dai YP, Miller L, Hume JR, Yamboliev IA (2007) Hypotonic activation of short ClC3 isoform is modulated by direct interaction between its cytosolic C-terminal tail and subcortical actin filaments. J Biol Chem 282:16871–16877
Mitchell CH, Zhang JJ, Wang L, Jacob TJ (1997) Volume-sensitive chloride current in pigmented ciliary epithelial cells: role of phospholipases. Am J Physiol 272:C212–C222
Miwa A, Ueda K, Okada Y (1997) Protein kinase C-independent correlation between P-glycoprotein expression and volume sensitivity of Cl− channel. J Membr Biol 157:63–69
Nilius B, Droogmans G (2003) Amazing chloride channels: an overview. Acta Physiol Scand 177:119–147
Nilius B, Oike M, Zahradnik I, Droogmans G (1994) Activation of a Cl− current by hypotonic volume increase in human endothelial cells. J Gen Physiol 103:787–805
Nilius B, Eggermont J, Voets T, Droogmans G (1996) Volume-activated Cl− channels. Gen Pharmacol 27:1131–1140
Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G (1997) Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol 68:69–119
Oike M, Schwarz G, Sehrer J, Jost M, Gerke V, Weber K, Droogmans G, Nilius B (1994) Cytoskeletal modulation of the response to mechanical stimulation in human vascular endothelial cells. Pflugers Arch 428:569–576
Okada Y (1997) Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol 273:C755–C789
Okada Y, Sato K, Numata T (2009) Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J Physiol 587:2141–2149
Olsen ML, Schade S, Lyons SA, Amaral MD, Sontheimer H (2003) Expression of voltage-gated chloride channels in human glioma cells. J Neurosci 23:5572–5582
Onesto C, Shutes A, Picard V, Schweighoffer F, Der CJ (2008) Characterization of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. Methods Enzymol 439:111–129
Ransom CB, O’Neal JT, Sontheimer H (2001) Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J Neurosci 21:7674–7683
Reetz G, Reiser G (1996) [Ca2+]i oscillations induced by bradykinin in rat glioma cells associated with Ca2+ store-dependent Ca2+ influx are controlled by cell volume and by membrane potential. Cell Calcium 19:143–156
Ren Z, Raucci FJ Jr, Browe DM, Baumgarten CM (2008) Regulation of swelling-activated Cl− current by angiotensin II signalling and NADPH oxidase in rabbit ventricle. Cardiovasc Res 77:73–80
Robson L, Hunter M (1994) Role of cell volume and protein kinase C in regulation of a Cl− conductance in single proximal tubule cells of Rana temporaria. J Physiol 480:1–7
Rondé P, Giannone G, Gerasymova I, Stoeckel H, Takeda K, Haiech J (2000) Mechanism of calcium oscillations in migrating human astrocytoma cells. Biochim Biophys Acta 1498:273–280
Schwiebert EM, Mills JW, Stanton BA (1994) Actin-based cytoskeleton regulates a chloride channel and cell volume in a renal cortical collecting duct cell line. J Biol Chem 269:7081–7089
Seitz RJ, Wechsler W (1987) Immunohistochemical demonstration of serum proteins in human cerebral gliomas. Acta Neuropathol 73:145–152
Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK (2002) Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91:406–413
Shimizu T, Numata T, Okada Y (2004) A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl− channel. Proc Natl Acad Sci USA 101:6770–6773
Shutes A, Onesto C, Picard V, Leblond B, Schweighoffer F, Der CJ (2007) Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J Biol Chem 282:35666–35678
Sontheimer H (2008) An unexpected role for ion channels in brain tumor metastasis. Exp Biol Med (Maywood) 233:779–791
Soroceanu L, Manning TJ Jr, Sontheimer H (1999) Modulation of glioma cell migration and invasion using Cl− and K+ ion channel blockers. J Neurosci 19:5942–5954
Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bösl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ (2001) Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron 29:185–196
Sundaresan M, Yu ZX, Ferrans VJ, Sulciner DJ, Gutkind JS, Irani K, Goldschmidt-Clermont PJ, Finkel T (1996) Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem J 318:379–382
Szücs G, Heinke S, De Greef C, Raeymaekers L, Eggermont J, Droogmans G, Nilius B (1996) The volume-activated chloride current in endothelial cells from bovine pulmonary artery is not modulated by phosphorylation. Pflugers Arch 431:540–548
Takai Y, Sasaki T, Tanaka K, Nakanishi H (1995) Rho as a regulator of the cytoskeleton. Trends Biochem Sci 20:227–231
Tilly BC, Kansen M, van Gageldonk PG, van den Berghe N, Galjaard H, Bijman J, de Jonge HR (1991) G-proteins mediate intestinal chloride channel activation. J Biol Chem 266:2036–2040
Tolias KF, Couvillon AD, Cantley LC, Carpenter CL (1998) Characterization of a Rac1- and RhoGDI-associated lipid kinase signaling complex. Mol Cell Biol 18:762–770
Tysnes BB, Mahesparan R (2001) Biological mechanisms of glioma invasion and potential therapeutic targets. J Neurooncol 53:129–147
Vanoye CG, Castro AF, Pourcher T, Reuss L, Altenberg GA (1999) Phosphorylation of P-glycoprotein by PKA and PKC modulates swelling-activated Cl− currents. Am J Physiol 276:C370–C378
Vignais PV (2002) The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci 59:1428–1459
Voets T, Manolopoulos V, Eggermont J, Ellory C, Droogmans G, Nilius B (1998) Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins. J Physiol 506:341–352
Wang L, Chen L, Jacob TJ (2000) The role of ClC-3 in volume-activated chloride currents and volume regulation in bovine epithelial cells demonstrated by antisense inhibition. J Physiol 524:63–75
Wang X, Devaiah SP, Zhang W, Welti R (2006) Signaling functions of phosphatidic acid. Prog Lipid Res 45:250–278
Zhang Y, Du G (2009) Phosphatidic acid signaling regulation of Ras superfamily of small guanosine triphosphatases. Biochim Biophys Acta 1791:850–855
Zholos A, Beck B, Sydorenko V, Lemonnier L, Bordat P, Prevarskaya N, Skryma R (2005) Ca2+- and volume-sensitive chloride currents are differentially regulated by agonists and store-operated Ca2+ entry. J Gen Physiol 125:197–211
Acknowledgments
This work was supported by grants from Fondazione Cassa di Risparmio Perugia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Luigi Catacuzzeno and Antonio Michelucci have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Catacuzzeno, L., Michelucci, A., Sforna, L. et al. Identification of Key Signaling Molecules Involved in the Activation of the Swelling-Activated Chloride Current in Human Glioblastoma Cells. J Membrane Biol 247, 45–55 (2014). https://doi.org/10.1007/s00232-013-9609-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-013-9609-9