Abstract
Analysis of polar organic chemicals in the aquatic environment is exacerbated by the lack of suitable and widely applicable enrichment methods. In this work, we assessed the suitability of a novel combination of well-known solid-phase extraction (SPE) materials in one cartridge as well as an evaporation method and for the enrichment of 26 polar model substances (predominantly log D < 0) covering a broad range of physico-chemical properties in three different aqueous matrices. The multi-layer solid-phase extraction (mlSPE) and evaporation method were investigated for the recovery and matrix effects of the model substances and analyzed with hydrophilic interaction liquid chromatography-tandem mass spectrometry (HILIC-MS/MS). In total, 65% of the model substances were amenable (> 10% recovery) to the mlSPE method with a mean recovery of 76% while 73% of the model substances were enriched with the evaporation method achieving a mean recovery of 78%. Target and non-target screening comparison of both methods with a frequently used reversed-phase SPE method utilizing “hydrophilic and lipophilic balanced” (HLB) material was performed. Target analysis showed that the mlSPE and evaporation method have pronounced advantages over the HLB method since the HLB material retained only 30% of the model substances. Non-target screening of a ground water sample with the investigated enrichment methods showed that the median retention time of all detected features on a HILIC system decreased in the order mlSPE (3641 features, median tR 9.7 min), evaporation (1391, 9.3 min), HLB (4414, 7.2 min), indicating a higher potential of the described methods to enrich polar analytes from water compared with HLB-SPE.

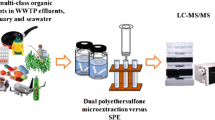
Schematic of the method evaluation (recovery and matrix effects) and method comparison (target and non-target analysis) of the two investigated enrichment methods for very polar chemicals in aqueousmatrices.





Similar content being viewed by others
References
Daughton CG. Non-regulated water contaminants: emerging research. Environ Impact Assess Rev. 2004;24(7–8):711–32.
Reemtsma T, Weiss S, Mueller J, Petrovic M, González S, Barcelo D, et al. Polar pollutants entry into the water cycle by municipal wastewater: a European perspective. Environ Sci Technol. 2006;40(17):5451–8.
Sjerps RMA, Vughs D, van Leerdam JA, ter Laak TL, van Wezel AP. Data-driven prioritization of chemicals for various water types using suspect screening LC-HRMS. Water Res. 2016;93:254–64.
Wirth O, Bunke D. PMT-Stoffe erkennen und ihre Emissionen vermeiden. https://www.umweltbundesamt.de/sites/default/files/medien/362/dokumente/2017_05_02_ridp_ws5_wasser_final.pdf. Last Access 07 2017.
Reemtsma T, Berger U, Arp HPH, Gallard H, Knepper TP, Neumann M, et al. Mind the gap: persistent and mobile organic compounds water contaminants that slip through. Environ Sci Technol. 2016;50(19):10308–15.
Ibáñez M, Pozo ÓJ, Sancho JV, López FJ, Hernández FJ. Re-evaluation of glyphosate determination in water by liquid chromatography coupled to electrospray tandem mass spectrometry. Chromatogr A. 2006;1134(1):51–5.
Quintana JB, Reemtsma T. Rapid and sensitive determination of ethylenediaminetetraacetic acid and diethylenetriaminepentaacetic acid in water samples by ion-pair reversed-phase liquid chromatography–electrospray tandem mass spectrometry. J Chromatogr A. 2007;1145(1):110–7.
Buszewski B, Noga S. Hydrophilic interaction liquid chromatography (HILIC)—a powerful separation technique. Anal Bioanal Chem. 2012;402(1):231–47.
Greco G, Grosse S, Letzel T. Serial coupling of reversed-phase and zwitterionic hydrophilic interaction LC/MS for the analysis of polar and nonpolar phenols in wine. J Sep Sci. 2013;36(8):1379–88.
Montes R, Aguirre J, Vidal X, Rodil R, Cela R, Quintana JB. Screening for polar chemicals in water by trifunctional mixed-mode liquid chromatography−high resolution mass spectrometry. Environ Sci Technol. 2017;51:6250–9.
Guillarme D, Veuthey J-L. Alternative strategies to reverse-phase liquid chromatography for the analysis of pharmaceutical compounds. Am Pharm Rev. 2017;20:46–51.
Scheurer M, Michel A, Brauch H-J, Ruck W, Sacher F. Occurrence and fate of the antidiabetic drug metformin and its metabolite guanylurea in the environment and during drinking water treatment. Water Res. 2012;46(15):4790–802.
Armbruster D, Happel O, Scheurer M, Harms K, Schmidt TC, Brauch HJ. Emerging nitrogenous disinfection byproducts: transformation of the antidiabetic drug metformin during chlorine disinfection of water. Water Res. 2015;79:104–18.
Knepper TP, Werner A, Bogenschütz G. Determination of synthetic chelating agents in surface and waste water by ion chromatography-mass spectrometry. J Chromatogr A. 2005;1085(2):240–6.
Yu Z, Jin F, Hu J, Zhang X, Sun J, Yang M. An improved method for analyzing chlormequat and mepiquat in source waters by solid-phase extraction and liquid chromatography-mass spectrometry. Anal Chim Acta. 2010;678(1):90–5.
Scheurer M, Storck FR, Graf C, Brauch H-J, Ruck W, Lev O, et al. Correlation of six anthropogenic markers in wastewater, surface water, bank filtrate, and soil aquifer treatment. J Environ Monit. 2011;13(4):966–73.
Zahn D, Frömel T, Knepper TP. Halogenated methanesulfonic acids: a new class of organic micropollutants in the water cycle. Water Res. 2016;101:292–9.
Huntscha S, Singer HP, McArdell CS, Frank CE, Hollender J. Multiresidue analysis of 88 polar organic micropollutants in ground, surface and wastewater using online mixed-bed multilayer solid-phase extraction coupled to high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1268:74–83.
Boos K-S, Fleischer C. Multidimensional on-line solid-phase extraction (SPE) using restricted access materials (RAM) in combination with molecular imprinted polymers (MIP). Fresenius J Anal Chem. 2001;371(1):16–20.
Castillo M, Alonso MC, Riu J, Barceló D. Identification of polar, ionic, and highly water soluble organic pollutants in untreated industrial wastewaters. Environ Sci Technol. 1999;33(8):1300–6.
Young, MS. Solid-phase extraction with Oasis® HLB sorbent: simple procedures for superior sample preparation. Operating manual; 1998.
Pluskal T, Castillo S, Villar-Briones A, Orešič M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics. 2010;11(1):395.
Chemicalize. Calculation. https://www.chemicalize.com. Accessed Nov 16 2017.
Scheurer M, Sacher F, Brauch H-J. Occurrence of the antidiabetic drug metformin in sewage and surface waters in Germany. J Environ Monit. 2009;11(9):1608–13.
Tran NH, Hu J, Ong SL. Simultaneous determination of PPCPs, EDCs, and artificial sweeteners in environmental water samples using a single-step SPE coupled with HPLC-MS/MS and isotope dilution. Talanta. 2013;113:82–92.
Longley D, Harkin D, Johnston P. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–8.
Golkiewicz W, Werkhoven-Goewie CE, Brinkman UAT, Frei RW, Colin H, Guiochon G. Use of pyrocarbon sorbents for trace enrichment of polar compounds from aqueous samples with on-line HPLC analysis. J Chromatogr Sci. 1983;21(1):27.
Liska I, Krupcík J, Leclercq PA. The use of solid sorbents for directaccumulation of organic compounds from water matricesa review of solid-phase extraction techniques. J High Resolut Chromatogr. 1989:577–90.
Funding
This work has been funded by the BMBF (02WU1347B) in the frame of the collaborative international consortium WATERJPI2013 - PROMOTE of the Water Challenges for a Changing World Joint Programming Initiative (Water JPI) Pilot Call.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 502 kb)
Rights and permissions
About this article
Cite this article
Köke, N., Zahn, D., Knepper, T.P. et al. Multi-layer solid-phase extraction and evaporation—enrichment methods for polar organic chemicals from aqueous matrices. Anal Bioanal Chem 410, 2403–2411 (2018). https://doi.org/10.1007/s00216-018-0921-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0921-1