Summary
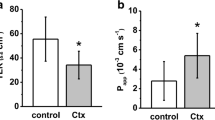
Epithelial cell tight junction structure in self-filling blind loops of rat jejunum, a model for blind loop syndrome in humans, was analyzed morphometrically along the crypt-villus axis. In control jejunum, the number of strands and junctional depth, including meshwork depth, decreased from crypt to villus tip. In the blind loop, aberrant strands appeared below the meshwork, particularly in crypt cells. Consequently, total junctional depth was greater than in controls. Furthermore, strand number and junctional meshwork depth were increased in blind loops at the villus tip. It is that site along the crypt-villus axis which showed the most shallow junction in control jejunum. This structural change is paralleled by a three-fold increase in epithelial resistance as previously measured by alternating current impedance analysis. Relative Na over Cl permeability (PNa:Cl) was obtained from dilution potential measurements. PNa:Cl was 1.50∶1 in control jejunum and 1.35∶1 in the blind loop (n.s.). Considering the cation selectivity of the tight junction, the increase in epithelial resistance in blind loops cannot be attributed to a collapse of the lateral intercellular space but is due to changes in tight junctional permeability resulting from structural alteration. The blind loop syndrome represents a further example of diminished epithelial ion transport and concomitant decrease in tight junction permeability, thus supporting the general concept of regulation of the tight junction in response to active transport activity.
Similar content being viewed by others
References
Bentzel CJ, Fromm M, Palant CE, Hegel U (1987) Protamine alters structure and conductance of Necturus gallbladder tight junctions without major effect on the apical membrane. J Membrane Biol 95:9–20
Bentzel CJ, Hainau B, Ho S, Hui SW, Edelman A, Anagnostopoulos T, Benedetti EL (1980) Cytoplasmic regulation of tight-junction permeability: effect of plant cytokinins. Am J Physiol 239:C75-C89
Bentzel CJ, Martinez M, Hainau B, Fromm M, Hegel U (1982) Morphological and physiological factors determining transjunctional fluxes. In: Bradley SE, Purcell EF (eds) The paracellular pathway. Josiah Macy Jr Foundation, New York, pp 275–286
Boulpaep EL (1972) Permeability changes of the proximal tubule of Necturus during saline loading. Am J Physiol 222:517–531
Claude P (1978) Morphological factors influencing transepithelial permeability: a model for the resistance of the zonula occludens. J Membrane Biol 39:219–232
Claude P, Goodenough DA (1973) Fracture faces of zonulae occludentes from tight and leaky epithelia. J Cell Biol 58:390–400
Duffey ME, Hainau B, Ho S, Bentzel CJ (1981) Regulation of epithelial tight junction permeability by cyclic AMP. Nature 294:451–453
Fromm M, Palant CE, Bentzel CJ, Hegel U (1985) Protamine reversibly decreases paracellular cation permeability in Necturus gallbladder. J Membrane Biol 87:141–150
Fromm M, Schulzke JD, Hegel U (1985) Epithelial and subepithelial contributions to transmural electrical resistance of intact rat jejunum, in vitro. Pflügers Arch 405:400–402
Gracey M, Papadimitriou J, Bower G (1974) Ultrastructural changes in the small intestines of rats with self-filling blind loops. Gastroenterology 67:646–651
Kottra G, Frömter E (1984) Rapid determination of intraepithelial resistance barriers by alternating current spectroscopy. II. Test of model circuits and quantification of results. Pflügers Arch 402:421–432
Krasny EJ, DiBona AI, Frizzell RA (1982) Regulation of paracellular permselectivity in flounder intestine. Bull Mount Des Isl Biol Lab 22:83–85
Lewy JE, Windhager EE (1968) Peritubular control of proximal tubular fluid reabsorption in the rat kidney. Am J Physiol 214:943–954
Luciano L, Reale E, Rechkemmer G, Engelhardt W v. (1984) Structure of zonulae occludentes and the permeability of the epithelium to short-chain fatty acids in the proximal and the distal colon of guinea pig. J Membrane Biol 82:145–156
Madara JL (1987) Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am J Physiol 253:C171–175
Madara JL (1983) Increases in guinea pig small intestinal transepithelial resistance induced by osmotic loads are accompanied by rapid alterations in absorptive-cell tight-junction structure. J Cell Biol 97:125–136
Madara JL, Barenberg D, Carlson S (1986) Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol 102:2125–2136
Madara JL, Pappenheimer JR (1987) Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membrane Biol 100:149–164
Madara JL, Trier JS, Neutra MR (1980) Structural changes in the plasma membrane accompanying differentiation of epithelial cells in human and monkey small intestine. Gastroenterology 78:963–975
Madara JL, Trier JS (1982) Structure and permeability of goblet cell tight junctions in rat small intestine. J Membrane Biol 66:145–157
Marcial MA, Carlson SL, Madara JL (1984) Partitioning of paracellular conductance along the ileal crypt-villus-axis: a hypothesis based on structural analysis with detailed consideration of tight junction structure-function relationships. J Membrane Biol 80:59–70
Martinez-Palomo A, Meza I, Beaty G, Cereijido M (1980) Experimental modulation of occluding junctions in a transporting epithelium. J Cell Biol 87:736–745
Maunsbach AB, Boulpaep EL (1980) Hydrostatic pressure changes related to paracellular shunt ultrastructure in proximal tubule. Kidney Int 17:732–748
Menge H, Köhn R, Dietermann KH, Lorenz-Mayer H, Riecken EO, Robinson JWL (1975) Structural and morphological alterations in the intestines of rats with an experimental blind loop syndrome. Res Exp Med 166:67–78
Menge H, Murer H (1983) Funktionelle Charakterisierung der Bürstensaummembranen selbstfüllender Blindschlingen des Rattenjejunums. Z Gastroenterol 21:381–382
Møllgård K, Malinowska DH, Saunders NR (1976) Lack of correlation between tight junction morphology and permeability properties in developing choroid plexus. Nature 264:293–294
Palant CE, Duffey ME, Mookerjee BK, Ho S, Bentzel CJ (1983) Regulation of tight junction permeability and structure in Necturus gallbladder. Am J Physiol 245:C203-C212
Robinson RA, Stokes RH (1959) Electrolyte solutions, 2nd edn. Academic, London
Schultz SG (1977) The role of paracellular pathways in isotonic fluid transport. Yale J Biol Med 50:99–113
Schulzke JD, Fromm M, Hegel U (1986) Epithelial and subepithelial resistance of rat large intestine: segmental differences, effect of stripping, time course, and action of aldosterone. Pflügers Arch 407:632–637
Schulzke JD, Fromm M, Menge H, Riecken EO (1987) Impaired intestinal sodium and chloride transport in the blind loop syndrome of the rat. Gastroenterology 92:693–698
Schulzke JD, Fromm M, Menge H, Riecken EO (1985) Der gestörte Elektrolyttransport im experimentellen Blindsacksyndrom. Schweiz Med Wochenschr 115:1032
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schulzke, J.D., Fromm, M., Zeitz, M. et al. Tight junction regulation during impaired ion transport in blind loops of rat jejunum. Res. Exp. Med. 190, 59–68 (1990). https://doi.org/10.1007/PL00020007
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/PL00020007