Abstract
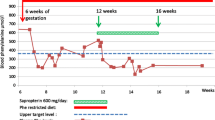
The Maternal Phenylketonuria Study began in 1984 and during the intervening years, 572 pregnancies in hyperphenylalaninemic women and 99 controls and their outcomes have been evaluated. Among hyperphenylalaninemic women who delivered a live infant, only 15.9% were treated and in metabolic control preconceptually, however, another 18.4% were in control by 10 weeks. Compared to the results reported by Lenke and Levy in 1980, there is a marked improvement in outcome with treatment. Microcephaly was unusual in preconceptually treated pregnancies with well controlled phenylalanine restricted diets. Even in pregnancies that established control after conception but before the 8th week, congenital heart disease did not occur in the offspring, however, it did occur in 12% of pregnancies not achieving control until after 10 weeks of pregnancy.
Conclusion The recommended level of blood phenylalanine during pregnancy is 120–360 μmol/l. Best results were obtained by close cooperation between the attending obstetrician and a metabolic team experienced in the care of persons with phenylketonuria.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koch, R., Friedman, E., Azen, C. et al. The international collaborative study of maternal phenylketonuria: status report 1998. Eur J Pediatr 159 (Suppl 2), S156–S160 (2000). https://doi.org/10.1007/PL00014383
Issue Date:
DOI: https://doi.org/10.1007/PL00014383