Abstract
Background
The MDM2 oncogene functions as a negative feedback regulator of the p53 tumor suppressor. Abnormal expression of MDM2 in tumors may attenuate the p53-mediated growth arrest and apoptosis response, resulting in increased cell proliferation and resistance to chemotherapy.
Materials and Methods
We have developed phosphorothioate antisense oligodeoxynucleotides optimized for inhibition of MDM2 expression and investigated the role of MDM2 in a large panel of tumor cell lines.
Results
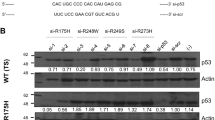
Inhibition of MDM2 expression in 15 tumor types containing wild-type p53 results in a significant induction of nuclear p53 accumulation. The increase in p53 level is due to prolonged half-life and is associated with an increase in p53 transcriptional activity, growth inhibition, or apoptosis. Inhibition of MDM2 expression is also sufficient to induce nuclear p53 accumulation in several cell lines with cytoplasmic p53.
Conclusions
The MDM2 negative feedback loop is important for maintenance of p53 at a low level by promoting p53 degradation. Nuclear export and degradation by MDM2 may contribute to the p53 nuclear exclusion phenotype. Inhibition of MDM2 expression can effectively activate p53 in most tumor types, including those without MDM2 overexpression, and may have broad anti-tumor potential.








Similar content being viewed by others
References
Levine AJ. (1997) p53, the cellular gatekeeper of growth and division. Cell 88: 323–331.
Hollstein M, Rice K, Greenblatt MS, et al. (1994) Database of p53 gene somatic mutations in human tumors and cell lines. Nucl. Acids Res. 22: 3551–3555.
Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. (1992) Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature 358: 80–83.
Cordon Cardo C, Latres E, Drobnjak M, et al. (1994) Molecular abnormalities of mdm2 and p53 genes in adult soft tissue sarcomas. Cancer Res. 54: 794–799.
Lowe SW, Ruley HE, Jacks T, Housman DE. (1993) p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74: 957–967.
Fakharzadeh SS, Trusko SP, George DL. (1991) Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 10: 1565–1569.
Finlay CA. (1993) The mdm-2 oncogene can overcome wild-type p53 suppression of transformed cell growth. Mol. Cell Biol. 13: 301–306.
Momand J, Zambetti GP, Olson DC, George D, Levine AJ. (1992) The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69: 1237–1245.
Haupt Y, Maya R, Kazaz A, Oren M. (1997) Mdm2 promotes the rapid degradation of p53. Nature 387: 296–299.
Kubbutat MHG, Jones SN, Vousden KH. (1997) Regulation of p53 stability by mdm2. Nature 387: 299–303.
Honda R, Tanaka H, Yasuda H. (1997) Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420: 25–27.
Wu X, Bayle JH, Olson D, Levine AJ. (1993) The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 7: 1126–1132.
Barak Y, Juven T, Haffner R, Oren M. (1993) mdm2 expression is induced by wild type p53 activity. EMBO J. 12: 461–468.
Oca Luna RM, Wagner DS, Lozano G. (1995) Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378: 203–206.
Jones SN, Roe AE, Donehower LA, Bradley A. (1995) Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 378: 206–208.
Midgley CA, Lane DP. (1997) p53 protein stability in tumour cells is not determined by mutation but is dependent on Mdm2 binding. Oncogene 15: 1179–1189.
Bottger A, Bottger V, Sparks A, Liu WL, Howard SF, Lane DP. (1997) Design of a synthetic Mdm2-binding mini protein that activates the p53 response in vivo. Curr. Biol. 7: 860–869.
Chen L, Agrawal S, Zhou W, Zhang R, Chen J. (1998) Synergistic activation of p53 by inhibition of mdm2 expression and DNA damage. Proc. Natl. Acad. Sci. U.S.A. 95: 195–200.
Chen J, Marechal V, Levine AJ. (1993) Mapping of the p53 and mdm-2 interaction domains. Mol. Cell Biol. 13: 4107–4114.
Takahashi K, Sumimoto H, Suzuki K, Ono T. (1993) Protein synthesis-dependent cytoplasmic translocation of p53 protein after serum stimulation of growth-arrested MCF-7 cells. Mol. Carcinogen 8: 58–66.
Moll UM, LaQuaglia M, Benard J, Riou G. (1995) Wild-type p53 protein undergoes cytoplasmic sequestration in undifferentiated neuroblastomas but not in differentiated tumors. Proc. Natl. Acad. Sci. U.S.A. 92: 4407–4411.
Goldman SC, Chen CY, Lansing TJ, Gilmer TM, Kastan MB. (1996) The p53 signal transduction pathway is intact in human neuroblastoma despite cytoplasmic localization. Am. J. Pathol. 148: 1381–1385.
Harvey DM, Levine AJ. (1991) p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 5: 2375–2385.
Rock KL, Gramm C, Rothstein L, et al. (1994) Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78: 761–771.
Chang YC, Lee YS, Tejima T, et al. (1998) mdm2 and bax, downstream mediators of the p53 response, are degraded by the ubiquitin-proteasome pathway. Cell Growth Differ. 9: 79–84.
Landers JE, Haines DS, Strauss JF, George DL. (1994) Enhanced translation: a novel mechanism of mdm2 oncogene overexpression identified in human tumor cells. Oncogene 9: 2745–2750.
Shieh SY, Ikeda M, Taya Y, Prives C. (1997) DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91: 325–334.
Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. (1992) Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc. Natl. Acad. Sci. U.S.A. 89: 7491–7495.
Nelson WG, Kastan MB. (1994) DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol. Cell Biol. 14: 1815–1823.
Shaulian E, Resnitzky D, Shifman O, et al. (1997) Induction of Mdm2 and enhancement of cell survival by bFGF. Oncogene 15: 2717–2725.
Moll UM, Riou G, Levine AJ. (1992) Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc. Natl. Acad. Sci. U.S.A. 89: 7262–7266.
Moll UM, Ostermeyer AG, Haladay R, Winkfield B, Frazier M, Zambetti G. (1996) Cytoplasmic sequestration of wild-type p53 protein impairs the G1 checkpoint after DNA damage. Mol. Cell Biol. 16: 1126–1137.
Roth J, Dobbelstein M, Freedman DA, Shenk T, Levine AJ. (1998) Nucleo-cytoplasmic shuttling of the hdm2-oncoprotein regulates the levels of p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 17: 554–564.
Clarke AR, Gledhill S, Hooper ML, Bird CC, Wyllie AH. (1994) p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following gamma-irradiation. Oncogene 9: 1767–1773.
Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. (1993) p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362: 847–849.
Gottlieb E, Haffner R, King A, et al. (1997) Transgenic mouse model for studying the transcriptional activity of the p53 protein: age- and tissue-dependent changes in radiation-induced activation during embryogenesis. EMBO J. 16: 1381–1390.
Komarova EA, Chernov MV, Franks R, et al. (1997) Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. EMBO J. 16: 1391–1400.
Acknowledgments
We are particularly grateful to Dr. Jay Hunt for use of the fluorescence microscope. We also thank Joelle Finley, Dr. James Gnarra, and Dr. Om Prakash for providing cell lines, and Dr. Ronald Luftig for critical reading of the manuscript. This work was supported by funding from the Stanley S. Scott Cancer Center and a grant from the American Cancer Society to J. Chen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Lu, W., Agrawal, S. et al. Ubiquitous Induction of p53 in Tumor Cells by Antisense Inhibition of MDM2 Expression. Mol Med 5, 21–34 (1999). https://doi.org/10.1007/BF03402136
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402136