Abstract
Background
The pathogenesis of bilirubin encephalopathy and Alzheimer’s disease appears to result from accumulation of unconjugated bilirubin (UCB) and amyloid-β (Aβ) peptide, respectively, which may cause apoptosis. Permeabilization of the mitochondrial membrane, with release of intermembrane proteins, has been strongly implicated in cell death. Inhibition of the mitochondrial permeability is one pathway by which ursodeoxycholate (UDC) and tauroursodeoxycholate (TUDC) protect against apoptosis in hepatic and nonhepatic cells. In this study, we further characterize UCB- and Aβ-induced cytotoxicty in isolated neural cells, and investigate membrane perturbation during incubation of isolated mitochondria with both agents. In addition, we evaluate whether the anti-apoptotic drugs UDC and TUDC prevent any changes from occurring.
Materials and Methods
Primary rat neuron and astrocyte cultures were incubated with UCB or Aβ peptide, either alone or in the presence of UDC. Apoptosis was assessed by DNA fragmentation and nuclear morphological changes. Isolated mitochondria were treated with each toxic, either alone or in combination with UDC, TUDC, or cyclosporine A. Mitochondrial swelling was measured spectrophotometrically and cytochrome c protein levels determined by Western blot.
Results
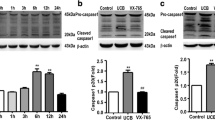
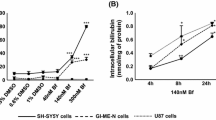
Incubation of neural cells with both UCB and Aβ induced apoptosis (p < 0.01). Coincubation with UDC reduced apoptosis by >50% (p < 0.05). Both toxins caused membrane permeabilization in isolated mitochondria (p < 0.001); whereas, pretreatment with UDC was protective (p < 0.05). TUDC was even more effective at preventing matrix swelling mediated by Aβ (p < 0.01). UDC and TUDC markedly reduced cytochrome c release associated with mitochondrial permeabilization induced by UCB and Aβ, respectively (p < 0.05). Moreover, cyclosporine A significantly inhibited mitochondrial swelling and cytochrome c efflux mediated by UCB (p < 0.05).
Conclusion
UCB and Aβ peptide activate the apoptotic machinery in neural cells. Toxicity occurs through a mitochondrial-dependent pathway, which in part involves opening of the permeability transition pore. Furthermore, membrane permeabilization is required for cytochrome c release from mitochondria and can be prevented by UDC or TUDC. These data suggest that the mitochondria is a pharmacological target for cytoprotection during unconjugated hyperbilirubinemia and neurodegenerative disorders, and that UDC or TUDC may be potential therapeutic agents.





Similar content being viewed by others
References
Jacobson MD, Weil M, Raff MC. (1997) Programmed cell death in animal development. Cell 88: 347–354.
Thompson CB. (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267: 1456–1462.
Evan G, Littlewood T. (1998) A matter of life and cell death. Science 281: 1317–1322.
Wyllie AH, Kerr JFR, Currie AR. (1980) Cell death: The significance of apoptosis. Int. Rev. Cytol. 68: 251–306.
Columbano A. (1995) Cell death: current difficulties in discriminating apoptosis from necrosis in the context of pathological processes in vivo. J. Cell Biochem. 58: 181–190.
Kroemer G, Zamzami N, Susin SA. (1997) Mitochondrial control of apoptosis. Immunol Today. 18: 44–51.
Green DR, Reed JC. (1998) Mitochondria and apoptosis. Science 281: 1309–1312.
Wallace DC. (1999) Mitochondrial diseases in man and mouse. Science 283: 1482–1488.
Jacobson MD, Burne JF, Raff MC. (1994) Programmed cell death and Bcl-2 protection in the absence of a nucleus. EMBO J. 13: 1899–1910.
Schulze-Osthoff K, Walczak H, Droge W, Krammer PH. (1994) Cell nucleus and DNA fragmentation are not required for apoptosis. J. Cell Biol. 127: 15–20.
Jacotot E, Costantini P, Laboureau E, Zamzami N, Susin SA, Kroemer G. (1999) Mitochondrial membrane permeabilization during the apoptotic process. Ann. NY Acad. Sci. 887: 18–30.
Brodersen R, Stern L. (1990) Deposition of bilirubin acid in the central nervous—a hypothesis for the development of kernicterus. Acta Paediatr. Scand. 79: 12–19.
Rubboli G, Ronchi F, Cecchi P, et al. (1997) A neurophysiological study in children and adolescents with Crigler-Najjar syndrome type I. Neuropediatrics 28: 281–286.
Majumadar APN. (1974) Bilirubin encephalopathy: effect on RNA polymerase activity and chromatin template activity in the brain of Gunn rat. Neurobiology 4: 425–431.
Yamada N, Sawasaki Y, Nakajima H. (1977) Impairment of DNA synthesis in Gunn rat cerebellum. Brain Res. 126: 295–307.
Ohno T. (1980) Kernicterus: effect on choline acetyltransferase, glutamic acid decarboxylase and tyrosine hydroxylase activities in the brain of Gunn rat. Brain Res. 196: 282–285.
Morphis L, Constantopoulos A, Matsaniotis N. (1982) Bilirubin-induced modulation of cerebral protein phosphorylation in neonate rabbits in vivo. Science 218: 156–158.
Sano K, Nakamura H, Matsuo T. (1982) Mode of inhibitory action of bilirubin on protein kinase C. Pediatr. Res. 19: 587–590.
Schiff D, Chan G, Poznansky MJ. (1985) Bilirubin toxicity in neuronal cell lines N-115 and NBR-10A. Pediatr. Res. 19: 908–911.
Hansen TWR, Bratlid D, Walaas SI. (1988) Bilirubin decreases phosphorylation of synapsin I, a synaptic vesicle-associated neuronal phosphoprotein, in intact synaptosomes from rat cerebral cortex. Pediatr. Res. 23: 219–223.
Silva R, Mata LR, Gulbenkian S, Brito MA, Tiribelli C, Brites D. (1999) Inhibition of glutamate uptake by unconjugated bilirubin in cultured cortical rat astrocytes: role of concentration and pH. Biochem. Biophys. Res. Comm. 265: 67–72.
Silva R, Rodrigues CMP, Brites D. (In press) Bilirubin-induced apoptosis in glial and nerve cells is aggravated by chenodeoxycholic acid but prevented by ursodeoxycholic acid. J. Hepatol.
Rodrigues CMP, Sola S, Silva R, Diógenes MJ, Brites D. (2000) Apoptosis induced by deoxycholic acid, unconjugated bilirubin and amyloid β-peptide reflects mitochondrial perturbation which may be inhibited by ursodeoxycholic acid (Abstract). J. Hepatol. 32: 40.
Cowger ML, Igo RP, Labbe RF. (1965) The mechanism of bilirubin toxicity studied with purified respiratory enzyme and tissue culture systems. Biochemistry 4: 2763–2770.
Mustafa MG, Cowger ML, King TE. (1969) Effects of bilirubin on mitochondrial reactions. J. Biol. Chem. 244: 6403–6414.
Noir BA, Boveris A, Garaza Pereira AM, Stoppani AO. (1972) Bilirubin: a multi-site inhibitor of mitochondrial respiration. FEBS Lett. 27: 270–274.
Glenner GG. (1988) Alzheimer’s disease: its proteins and genes. Cell 52: 307–308.
Yankner BA, Duffy LK, Kirschner DA. (1990) Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science 250: 279–282.
Behl C, Davis J, Cole GM, Schubert D. (1992) Vitamin E protects nerve cells from amyloid beta protein toxicity. Biochem. Biophys. Res. Commun. 186: 944–950.
Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. (1992) β-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 12: 376–389.
Su JH, Anderson AJ, Cummings BJ, Cotman CW. (1994) Immunohistochemical evidence for apoptosis in Alzheimer’s disease. Neuroreport 5: 2529–2533.
Cotman CW, Anderson AJ. (1995) A potential role for apoptosis in neurodegeneration and Alzheimer’s disease. Mol. Neurobiol. 10: 19–45.
Rodrigues CMP, Fan G, Ma X, Kren BT, Steer CJ. (1998) A novel role of ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J. Clin. Invest. 101: 2790–2799.
Rodrigues CMP, Ma X, Linehan-Stieers C, Fan G, Kren BT, Steer CJ. (1999) Ursodeoxycholic acid prevents cytochrome c release in apoptosis by inhibiting mitochondrial membrane depolarization and channel formation. Cell Death Differ. 6: 842–854.
Rodrigues CMP, Keene CD, Linehan-Stieers C, Ma X, Low W, Steer CJ. (2000) Tauroursodeoxycholic acid prevents apoptosis induced by the neurotoxin 3-nitropropionic acid in rat neuronal cells: evidence for a mitochondrial-dependent pathway that does not involve the permeability transition (Abstract). J. Hepatol. 32: 86.
Brewer GJ, Torricelli JR, Evege EK, Price PJ. (1993) Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 35: 567–576.
Blondeau JP, Beslin A, Chantoux F, Francon J. (1993) Triiodothyronine is a high-affinity inhibitor of amino acid transport system L1 in cultured astrocytes. J. Neurochem. 60: 1407–1413.
McDonagh AF, Assisi F. (1972) The ready isomerization of bilirubin IX- in aqueous solution. Biochem. J. 129: 797–800.
Oberhammer FA, Pavelka M, Sharma S, et al. (1992) Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor β 1. Proc. Natl. Acad. Sci. USA 89: 5408–5412.
Walajtys-Rhode E, Zapatero J, Moehren G, Hoek JB. (1992) The role of the matrix calcium level in the enhancement of mitochondrial pyruvate carboxylation by glucagon pretreatment. J. Biol. Chem. 267: 370–379.
Botla R, Spivey JR, Aguilar H, Bronk SF, Gores GJ. (1995) Ursodeoxycholate (UDCA) inhibits the mitochondrial membrane permeability transition induced by glycochenodeoxycholate: a mechanism of UDCA cytoprotection. J. Pharmacol. Exp. Ther. 272: 930–938.
Sokol RJ, Devereaux M, Mierau GW, Hambidge KM, Shikes RH. (1990) Oxidant injury to hepatic mitochondrial lipids in rats with dietary copper overload. Modification by vitamin E deficiency. Gastroenterology 99: 1061–1071.
Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ. (1998) Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol. Med. 4: 165–178.
Amit Y, Brenner T. (1993) Age-dependent sensitivity of cultured rat glial cells to bilirubin toxicity. Exp. Neurol. 121: 248–255.
Hansen TW, Allen JW. (1997) Oxidation of bilirubin by brain mitochondrial membranes-dependence on Cell type and postnatal age. Biochem. Mol. Med. 60: 155–160.
Rhine WD, Schmitter SP, Yu AC, Eng LF, Stevenson DK. (1999) Bilirubin toxicity and differentiation of cultured astrocytes. J. Perinatol. 19: 206–211.
McLoughlin DJ, Howell ML. (1987) Bilirubin inhibition of enzymes involved in the mitochondrial malate-aspartate shuttle. Biochim. Biophys. Acta 893: 7–12.
Batty HK, Millhouse OE. (1976) Ultrastructure of the Gunn rat substantia nigra. II. Mitochondrial changes. Acta Neuropathol. 34: 7–19.
Kamisaka K, Gatmaitan Z, Moore CL, Arias IM. (1975) Ligandin reverses bilirubin inhibition of liver mitochondrial respiration in vitro. Pediatr. Res. 9: 903–905.
Mayor F. Diez-Guerra J, Valdivieso F, Mayor F. (1986) Effect of bilirubin on the membrane potential of rat brain synaptosomes. J. Neurochem. 47: 363–369.
Beal MF. (1998) Mitochondrial dysfunction in neurodegenerative diseases. Biochim. Biophys. Acta 1366: 211–223.
Christen Y. (2000) Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 71: 621S–629S.
Canevari L, Clark JB, Bates TE. (1999) β-Amyloid fragment 25–35 selectively decreases complex IV activity in isolated mitochondria. FEBS Lett. 457: 131–134.
Prasad KN, Cole WC, Hovland AR, et al. (1999) Multiple antioxidants in the prevention and treatment of neurodegenerative disease: analysis of biologic rationale. Curr. Opin. Neurol. 12: 761–770.
Kim CN, Wang X, Huang Y, et al. (1997) Over-expression of Bcl-XL inhibits Ara-C-induced mitochondrial loss of cytochrome c and other perturbations that activate the molecular cascade of apoptosis. Cancer Res. 57: 3115–3120.
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275: 1132–1136.
Yang J, Liu X, Bhalla K, et al. (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275: 1129–1132.
Neame SJ, Rubin LL, Philpott KL. (1998) Blocking cytochrome c activity within intact neurons inhibits apoptosis. J. Cell Biol. 142: 1583–1593.
Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. (2000) The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell Biol. 2: 156–162.
Bossy-Wetzel E, Newmeyer DD, Green DR. (1998) Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 17: 37–49.
Liu X, Kim CN, Yang J, Jemmerson R, Wang X. (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157.
Li P, Nijhawan D, Budihardjo I, et al. (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489.
Acknowledgements
This work was supported by grant PRAXIS/C/SAU/14311/1998 from Fundaçào para a Ciência e a Tecnologia, Lisbon, Portugal, and EASL Research Fellowship from the European Association for the Study of the Liver to CMP Rodrigues.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, C.M.P., Solá, S., Silva, R. et al. Bilirubin and Amyloid-β Peptide Induce Cytochrome c Release Through Mitochondrial Membrane Permeabilization. Mol Med 6, 936–946 (2000). https://doi.org/10.1007/BF03401828
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401828