Abstract
Background
Suberoylanilide hydroxamic acid (SAHA) is a prototype of the newly developed, second-generation, hybrid polar compounds. It is a novel histone deacetylase inhibitor with high potency for inducing cell differentiation of cultured murine erythroleukemia cells. Studies with SAHA have primarily been performed with hematopoietic tumor cells. Here we extent these studies with SAHA to human breast cancer cell lines in an attempt to find better therapeutic agents for breast cancer treatment.
Materials and Methods
Human breast cancer cell lines, MCF7, MDA-MB-231, and MDA-MB-435, as well as normal cells, including the normal breast epithelial cell line MCF-10A, and fibroblasts, were treated with SAHA. Cells assayed for cell survival by using trypan blue exclusion assay, colony formation assay, and cell cycle and apoptosis analysis. The effects of SAHA on cell cycle and apoptosis regulatory proteins were examined by Western blots analysis. The identification of additional target genes was carried out by differential display (DD) and reverse transcription-polymerase chain reaction (RT-PCR).
Results
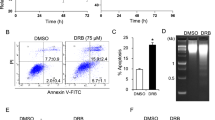
SAHA inhibited clonogenic growth of MCF7, MDA-MB-231, and MDA-MB-435 breast cancer cells. These cells were more sensitive to SAHA-mediated cytotoxic effects than normal breast epithelial cells and fibroblasts. The cytotoxic effects of SAHA on breast cancer cells were manifested by G1 and G2/M cell cycle arrest and eventual apoptosis. The pan-caspase inhibitor, Z-VAD.fmk, blocked SAHA-induced cell death, DNA laddering, and cleavage of poly(ADP-ribose) polymerase, indicating the involvement of caspases in SAHA-mediated apoptosis. In addition, SAHA modulated cell cycle and apoptosis regulatory proteins. For example, cyclin-dependent kinase (CDK) inhibitors p21WAF1/Cip1 and p27Kip1 were induced, and retinoblastoma protein pRb was hypophosphorylated. Moreover, SAHA induced several genes associated with differentiation and/or growth inhibition. These genes encode gelsolin, isopentenyl-diphosphate delta isomerase (IDI1), and 1,25-dihydroxyvitamin D-3 up-regulated protein 1 (VDUP1), the last two of which were identified by DD. Induction of these genes may contribute to SAHA-mediated pro-differentiating and antiproliferative effects.
Conclusions
SAHA induced growth inhibition, cell cycle arrest, and eventual apoptosis in human breast cancer cells, possibly by modulating cell cycle and apoptosis regulatory proteins, such as CDK inhibitors p21 and p27, pRb, and other differentiation and/or growth inhibition-associated genes, including gelsolin, IDI1 and VDUP1. This, together with the low toxicity in normal cells, suggests that SAHA might have therapeutic potential for the treatment of human breast cancers.








Similar content being viewed by others
References
Hortobagyi GN. (1998) Drug therapy—treatment of breast cancer. N. Engl. J. Med. 339: 974–984.
Holdener EE, Bollag W. (1993) Retinoids. Curr. Opin. Oncol. 5: 1059–1066.
Budd GT, Adamson PC, Gupta M, et al. (1998) Phase I/II trial of all-trans retinoic acid and tamoxifen in patients with advanced breast cancer. Clin. Cancer Res. 4: 635–642.
Richon VM, Webb Y, Merger R, et al. (1996) Second generation hybrid polar compounds are potent inducers of transformed cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 93: 5705–5708.
Richon VM, Russo P, Venta PG, Cordon CC, Rifkind RA, Marks PA. (1997) Two cytodifferentiation agent-induced pathways, differentiation and apoptosis, are distinguished by the expression of human papillomavirus 16 E7 in human bladder carcinoma cells. Cancer Res. 57: 2789–2798.
Siegel DS, Zhang X, Feinman R, et al. (1998) Hexamethylene bisacetamide induces programmed cell death (apoptosis) and down-regulates BCL-2 expression in human myeloma cells. Proc. Natl. Acad. Sci. U.S.A. 95: 162–166.
Andreeff M, Stone R, Michaeli J, et al. (1992) Hexamethylene bisacetamide in myelodysplastic syndrome and acute myelogenous leukemia: a phase II clinical trial with a differentiation-inducing agent. Blood 80: 2604–2609.
Vrana JA, Decker RH, Johnson CR, et al. (1999) Induction of apoptosis in U937 human leukemia cells by suberoylanilide hydroxamic acid (SAHA) proceeds through pathways that are regulated by Bcl-2/Bcl-XL, c-Jun, and p21CIP1, but independent of p53. Oncogene 18: 7016–7025.
Cohen LA, Amin S, Marks PA, Rifkind RA, Desai D, Richon VM. (1999) Chemoprevention of carcinogen-induced mammary tumorigenesis by the hybrid polar cytodifferentiation agent, suberanilohydroxamic acid (SAHA). Anticancer Res. 19: 4999–5005.
Cohen LA, Amin S, Marks PA, Rifkind RA, Desai D, Richon VM. (1999) Chemoprevention of carcinogen-induced mammary tumorigenesis by the hybrid polar cytodifferentiating agent, suberanilohydroxamic acid (SAHA). Anticancer Res. 19: 4999–5005.
Richon VM, Emiliani S, Verdin E, et al. (1998) A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc. Natl. Acad. Sci. U.S.A. 95: 3003–3007.
Yoshida M, Kijima M, Akita M, Beppu T. (1990) Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 265: 17174–17179.
Finnin MS, Donigian JR, Cohen A, et al. (1999) Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 401: 188–193.
Struhl K. (1998) Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 12: 599–606.
Wolffe AP. (1997) Transcriptional control. Sinful repression. Nature 387: 16–17.
Pazin MJ, Kadonaga JT. (1997) What’s up and down with histone deacetylation and transcription? Cell 89: 325–328.
Torchia J, Glass C, Rosenfeld MG. (1998) Co-activators and co-repressors in the integration of transcriptional responses. Curr. Opin. Cell Biol. 10: 373–383.
Magnaghi JL, Groisman R, Naguibneva I, et al. (1998) Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature 391: 601–605.
Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. (1998) Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature 391: 597–601.
Hassig CA, Fleischer TC, Billin AN, Schreiber SL, Ayer DE. (1997) Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell 89: 341–347.
Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN. (1997) Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell 89: 349–356.
Warrell RP, He LZ, Richon V, Calleja E, Pandolfi PP. (1998) Therapeutic targeting of transcription in acute promyelocytic leukemia by use of an inhibitor of histone deacetylase. J. Natl. Cancer Inst. 90: 1621–1625.
Lin RJ, Nagy L, Inoue S, Shao W, Miller WJ, Evans RM. (1998) Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature 391: 811–814.
Grignani F, De MS, Nervi C, et al. (1998) Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature 391: 815–818.
He LZ, Guidez F, Tribioli C, et al. (1998) Distinct interactions of PML-RARalpha and PLZF-RAR-alpha with co-repressors determine differential responses to RA in APL. Nat. Genet. 18: 126–135.
Alnemri ES, Livingston DJ, Nicholson DW, et al. (1996) Human ICE/CED-3 protease nomenclature. Cell 87: 171.
Salvesen GS, Dixit VM. (1997) Caspases: intracellular signaling by proteolysis. Cell 91: 443–446.
Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. (1993) Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 53: 3976–3985.
Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. (1994) Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 371: 346–347.
Bates S, Vousden KH. (1996) p53 in signaling checkpoint arrest or apoptosis. Curr. Opin. Genet. Dev. 6: 12–18.
O’Connor PM, Jackman J, Bae I, et al. (1997) Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 57: 4285–4300.
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75: 805–816.
Dulic V, Kaufmann WK, Wilson SJ, et al. (1994) p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 76: 1013–1023.
Kato JY, Matsuoka M, Polyak K, Massague J, Sherr CJ. (1994) Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell 79: 487–496.
Toyoshima H, Hunter T. (1994) p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78: 67–74.
Reed JC. (1994) Bcl-2 and the regulation of programmed cell death. J. Cell Biol. 124: 1–6.
Korsmeyer SJ. (1995) Regulators of cell death. Trends Genet. 11: 101–105.
Weinberg RA. (1995) The retinoblastoma protein and cell cycle control. Cell 81: 323–330.
Almasan A, Yin Y, Kelly RE, et al. (1995) Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proc. Natl. Acad. Sci. USA 92: 5436–5440.
Janicke RU, Walker PA, Lin XY, Porter AG. (1996) Specific cleavage of the retinoblastoma protein by an ICE-like protease in apoptosis. EMBO J. 15: 6969–6978.
Hahn FM, Xuan JW, Chambers AF, Poulter CD. (1996) Human isopentenyl diphosphate: di-methylallyl diphosphate isomerase: overproduction, purification, and characterization. Arch. Biochem. Biophys. 332: 30–34.
Xuan JW, Kowalski J, Chambers AF, Denhardt DT. (1994) A human promyelocyte mRNA transiently induced by TPA is homologous to yeast IPP isomerase. Genomics 20: 129–131.
Chen KS, DeLuca HF. (1994) Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim. Biophys. Acta 1219: 26–32.
Nishiyama A, Matsui M, Iwata S, et al. (1999) Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 274: 21645–21650.
Fujii S, Nanbu Y, Nonogaki H, et al. (1991) Co-expression of adult T-cell leukemia-derived factor, a human thioredoxin homologue, and human papillomavirus DNA in neoplastic cervical squamous epithelium. Cancer 68: 1583–1591.
Nakamura H, Masutani H, Tagaya Y, et al. (1992) Expression and growth-promoting effect of adult T-cell leukemia-derived factor. A human thioredoxin homologue in hepatocellular carcinoma. Cancer 69: 2091–2097.
Berggren M, Gallegos A, Gasdaska JR, Gasdaska PY, Warneke J, Powis G. (1996) Thioredoxin and thioredoxin reductase gene expression in human tumors and cell lines, and the effects of serum stimulation and hypoxia. Anticancer Res. 16: 3459–3466.
Wakasugi N, Tagaya Y, Wakasugi H, et al. (1990) Adult T-cell leukemia-derived factor/thioredoxin, produced by both human T-lymphotropic virus type I- and Epstein-Barr virus-transformed lymphocytes, acts as an autocrine growth factor and synergizes with interleukin 1 and interleukin 2. Proc. Natl. Acad. Sci. U.S.A. 87: 8282–8286.
Gasdaska JR, Berggren M, Powis G. (1995) Cell growth stimulation by the redox protein thioredoxin occurs by a novel helper mechanism. Cell Growth Differ. 6: 1643–1650.
Gallegos A, Gasdaska JR, Taylor CW, et al. (1996) Transfection with human thioredoxin increases cell proliferation and a dominantnegative mutant thioredoxin reverses the transformed phenotype of human breast cancer cells. Cancer Res. 56: 5765–5770.
Tanaka M, Mullauer L, Ogiso Y, et al. (1995) Gelsolin: a candidate for suppressor of human bladder cancer. Cancer Res. 55: 3228–3232.
Asch HL, Head K, Dong Y, et al. (1996) Widespread loss of gelsolin in breast cancers of humans, mice, and rats. Cancer Res. 56: 4841–4845.
Dosaka AH, Hommura F, Fujita H, et al. (1998) Frequent loss of gelsolin expression in non-small cell lung cancers of heavy smokers. Cancer Res. 58: 322–327.
Lee HK, Driscoll D, Asch H, Asch B, Zhang PJ. (1999) Downregulated gelsolin expression in hyperplastic and neoplastic lesions of the prostate. Prostate 40: 14–19.
Asch HL, Winston JS, Edge SB, Stomper PC, Asch BB. (1999) Down-regulation of gelsolin expression in human breast ductal carcinoma in situ with and without invasion. Breast Cancer Res. Treat. 55: 179–188.
Dong Y, Asch HL, Medina D, et al. (1999) Concurrent deregulation of gelsolin and cyclin D1 in the majority of human and rodent breast cancers. Int. J. Cancer 81: 930–938.
Mielnicki LM, Ying AM, Head KL, Asch HL, Asch BB. (1999) Epigenetic regulation of gelsolin expression in human breast cancer cells. Exp. Cell Res. 249: 161–176.
Schwartz SB, Higgins PJ, Rajasekaran AK, Staiano CL. (1994) Gelsolin expression in normal human keratinocytes is a function of induced differentiation. Adv. Exp. Med. Biol. 358: 169–181.
Olsen E, Rasmussen HH, Celis JE. (1995) Identification of proteins that are abnormally regulated in differentiated cultured human keratinocytes. Electrophoresis 16: 2241–2248.
Dieffenbach CW, SenGupta DN, Krause D, Sawzak D, Silverman RH. (1989) Cloning of murine gelsolin and its regulation during differentiation of embryonal carcinoma cells. J. Biol. Chem. 264: 13281–13288.
Qiu L, Kelso MJ, Hansen C, West ML, Fairlie DP, Parsons PG. (1999) Anti-tumour activity in vitro and in vivo of selective differentiating agents containing hydroxamate. Br. J. Cancer 80: 1252–1258.
Richon VM, Rifkind RA, Marks PA. (1992) Expression and phosphorylation of the retinoblastoma protein during induced differentiation of murine erythroleukemia cells. Cell Growth Differ. 3: 413–420.
Nicholson DW, Thornberry NA. (1997) Caspases: killer proteases. Trends Biochem. Sci. 22: 299–306.
Porter AG, Janicke RU. (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 6: 99–04.
Janicke RU, Sprengart ML, Wati MR, Porter AG. (1998) Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 273: 9357–9360.
Woo M, Hakem R, Soengas MS, et al. (1998) Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 12: 806–819.
Gooch JL, Yee D. (1999) Strain-specific differences in formation of apoptotic DNA ladders in MCF-7 breast cancer cells. Cancer Lett. 144: 31–37.
Orth K, Chinnaiyan AM, Garg M, Froelich CJ, Dixit VM. (1996) The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J. Biol. Chem. 271: 16443–16446.
Germain M, Affar EB, D’Amours D, Dixit VM, Salvesen GS, Poirier GG. (1999) Cleavage of au-tomodified poly(ADP-ribose) polymerase during apoptosis—Evidence for involvement of caspase-7. J. Biol. Chem. 274: 28379–28384.
Dittmer D, Pati S, Zambetti G, et al. (1993) Gain of function mutations in p53. Nat. Genet. 4: 42–46.
Liu PK, Kraus E, Wu TA, Strong LC, Tainsky MA. (1996) Analysis of genomic instability in Li-Fraumeni fibroblasts with germline p53 mutations. Oncogene 12: 2267–2278.
Lowe SW, Bodis S, McClatchey A, et al. (1994) p53 status and the efficacy of cancer therapy in vivo. Science 266: 807–810.
Lowe SW. (1995) Cancer therapy and p53. Curr. Opin. Oncol. 7: 547–553.
Nakano K, Mizuno T, Sowa Y, et al. (1997) Butyrate activates the WAF1/Cip1 gene promoter through Sp1 sites in a p53-negative human colon cancer cell line. J. Biol. Chem. 272: 22199–22206.
Archer SY, Meng S, Shei A, Hodin RA. (1998) p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc.Natl. Acad. Sci. U.S.A. 95: 6791–6796.
Sowa Y, Orita T, Minamikawa S, et al. (1997) Histone deacetylase inhibitor activates the WAF1/Cip1 gene promoter through the Sp1 sites. Biochem. Biophy. Res. Commun. 241: 142–150.
Sambucetti LC, Fischer DD, Zabludoff S, et al. (1999) Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and antiproliferative effects. J. Biol. Chem. 274: 34940–34947.
Olson MF, Marais R. (2000) Ras protein signalling. Semin. Immunol. 12: 63–73.
Katagiri K, Hattori S, Nakamura S, Yamamoto T, Yoshida T, Katagiri T. (1994) Activation of Ras and formation of GAP complex during TPA-induced monocytic differentiation of HL-60 cells. Blood 84: 1780–1789.
Adachi M, Ryo R, Yoshida A, et al. (1992) Induction of smg p21/rap1A p21/krev-1 p21 gene expression during phorbol ester-induced differentiation of a human megakaryocytic leukemia cell line. Oncogene 7: 323–329.
Young LH, Yang X, Voigt JM. (1996) Alteration of gene expression in rat mammary tumors induced by N-methyl-N-nitrosourea. Mol. Carcinog. 15: 251–260.
Yang X, Young LH, Voigt JM. (1998) Expression of a vitamin D-regulated gene (VDUP-1) in untreated- and MNU-treated rat mammary tissue. Breast Cancer Res. Treat. 48: 33–44.
Tagaya Y, Maeda Y, Mitsui A, et al. (1989) ATL-derived factor (ADF), an IL-2 receptor/Tac inducer homologous to thioredoxin; possible involvement of dithiol-reduction in the IL-2 receptor induction. EMBO J. 8: 757–764.
Kwiatkowski DJ. (1988) Predominant induction of gelsolin and actin-binding protein during myeloid differentiation. J. Biol. Chem. 263: 13857–13862.
Tanaka M, Sazawa A, Shinohara N, et al. (1999) Gelsolin gene therapy by retrovirus producer cells for human bladder cancer in nude mice. Cancer Gene Ther. 6: 482–487.
Hoshikawa Y, Kwon HJ, Yoshida M, Horinouchi S, Beppu T. (1994) Trichostatin A induces morphological changes and gelsolin expression by inhibiting histone deacetylase in human carcinoma cell lines. Exp. Cell Res. 214: 189–197.
Kim YB, Lee KH, Sugita K, Yoshida M, Horinouchi S. (1999) Oxamflatin is a novel antitumor compound that inhibits mammalian histone deacetylase. Oncogene 18: 2461–2470.
Saito A, Yamashita T, Mariko Y, et al. (1999) A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. Proc. Natl. Acad. Sci. U.S.A. 96: 4592–4597.
Nan X, Ng HH, Johnson CA, et al. (1998) Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393: 386–389.
Jones PL, Veenstra GJ, Wade PA, et al. (1998) Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 19: 187–191.
Acknowledgments
We thank Drs. Victoria M. Richon and Paul A. Marks for generously providing (CBHA) and SAHA, and Dr. Heide Ford for critical reading of the manuscript. This work was supported by an NIH grant RO1CA61253 (to A.B.P.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Pardee.
Rights and permissions
About this article
Cite this article
Huang, L., Pardee, A.B. Suberoylanilide Hydroxamic Acid as a Potential Therapeutic Agent for Human Breast Cancer Treatment. Mol Med 6, 849–866 (2000). https://doi.org/10.1007/BF03401823
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401823