Summary
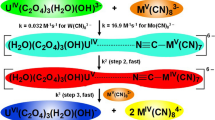
The kinetics of the oxidation of iodide by diaquotetrakis-(2,2-bipyridine)-μ-oxodiruthenium(III), [Ru2O]4+, were studied in aqueous perchloric acid at 25.0±0.1°C, I = 1.0 M (NaClO4). The reaction conforms to the overall equation: $$ {⤪ [Ru_{2}O]^{4+}+I^{-}⌝ghtarrow [Ru_{2}O]^{3+}+{1⩈er 2}I_{2}} $$ The experimental data are consistent with a second-order rate law: $$ {⤪ -d[Ru_{2}O^{4+}]/dt=k_{2}[Ru_{2}O^{4+}][I^{-}]} $$ with k2 = (3.5 ± 0.2) × 10−2 M−1 s−1 at [H+] = 0.05 M. Added Li+, K+, Na+, Mg2+, Zn2+, Cl−, Br− and $ {⤪ NO_{3}^{-}} $ had no effect on the rate of the reaction. The reaction rate was independent of variation in the ionic strength of the reaction medium in the range 0.25 ⩽ I ⩽ 2.0 M, but linearly dependent on [H+] in the acid range 0.05 ⩽ [H+] ⩽ 1.0 M. The absence of both kinetic and spectroscopic evidence for complex formation suggests that the reaction proceeds by the outer-sphere mechanism; this deduction is further supported by the non-conformity of the rate data to the Michaelis-Menten equation.
Similar content being viewed by others
References
D. M. Kern and C. H. Kim, J. Amer. Chem. Soc., 87, 5309 (1965).
K. J. Morgan, M. G. Peard and C. F. Cullis, J. Chem. Soc., 1865 (1965).
K. J. Morgan, Quarterly Review, 8, 123 (1954).
D. E. Myers and J. W. Kennedy, J. Amer. Chem. Soc., 72, 879 (1950).
A. Indelli, F. Ferranti and F. Secco, J. Phys. Chem., 70, 631 (1966).
L. J. Kirschenbaum and J. R. Sutter, J. Phys. Chem., 70, 3863 (1966).
A.J. Fudge and K. W. Sykes, J. Chem. Soc., 119 (1952).
K. W. Sykes, J. Chem. Soc., 124 (1952).
Y. A. Majid and K. E. Howlett, J. Chem. Soc. (A), 679 (1968).
N. K. Shastri, T. O. Wear and E. S. Amis, J. Inorg. Nucl. Chem., 24, 535 (1962).
M. H. Ford-Smith and J. H. Rawsthrone, J. Chem. Soc. (A), 160 (1969).
F. Ferranti, J. Chem. Soc. (A), 134 (1970).
A. McAuley, Inorg. Chem., 3, 719 (1980).
M. A. Olatunji and G. A. Ayoko, Polyhedron, 4, 191 (1984).
G. Nord, B. Pedersenand and O. Farver, Inorg. Chem., 17, 2233 (1978).
C. O. Adedinsewo and A. Adegite, Inorg. Chem., 18, 3597 (1979).
J. Ige, J. F. Ojo and O. Olubuyide, Can. J. Chem., 57, 2065 (1979).
T. R. Ray, T. J. Meyer, S. A. Adeyemi, G. M. Brown, P. P. Eckberg, W. E. Hatfield, E. C. Johnson, R. W. Murray and D. Unterker, J. Amer. Chem. Soc., 97, 3039 (1975).
Taqui-Khan, M. M. Ramachandraiah and Prakash Rao, Inorg. Chem., 25, 665 (1986).
C. Crentz and N. Sutin, Proc. Natl. Acad. Sci. USA, 72, 2858 (1975).
C. Chi-Ming, Z.N. Kwok, L. Wai-Ho and P. Chung- Kwong, Inorg. Chem., 25, 345 (1986).
J. T. Meyer, K. B. Sipe and B. A. Meyer, Inorg. Chem., 20, 1475 (1981).
J. Copper, W. D. Reents, Jr. M. Woods, R. Sjoblon and J. C. Sullivan, Inorg. Chem., 16, 1030 (1977).
G. A. Ayoko, M.Sc. Thesis, A. B. U. Zaria, Nigeria (1981).
J. F. Iyun, J. Chem. Soc. Nigeria, in press.
G. A. Ayoko and M. A. Olatunji, Transition Met. Chem., 10, 218 (1985).
K. K. Sen Gupta, S. Das and S. Sen-Gupta, Transition Met. Chem., 12, 417 (1987).
A. Adegite, J. Femi Iyun and J. F. Ojo, J. Chem. Soc., Dalton Trans., 115 (1977).
T. J. Przystas and N. Sutin, J. Amer. Chem. Soc., 95, 5545 (1973).
V. Henri, Compt. Renal. Acad. Sci., Paris, 135, 916 (1902)
L. Michaelis and M. L. Menten, Biochem. Z., 49, 333 (1913).
Author information
Authors and Affiliations
Additional information
Authors to whom all correspondence should be directed.
Rights and permissions
About this article
Cite this article
Iyun, J.F., Ayoko, G.A. & Lawal, H.M. Kinetics and mechanism of the oxidation of iodide by diaquote- trakis(2,2′-bipyridine)-μ-oxodiruthenium(III) ion in acid medium. Transition Met Chem 17, 63–65 (1992). https://doi.org/10.1007/BF03325418
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03325418