Summary
The metabolism of propafenone was studied in three humans by using 300 mg of the deuterated compound given orally. The excretion of propafenone and its metabolites was assessed by measuring the deuterium content in the excretion products by means of a microwave plasma detector. At selected times the metabolic pattern in plasma, urine, bile and feces was determined. The metabolites were isolated and the structure of the main compounds was characterized.
Propafenone is absorbed completely and quantitatively metabolized. Unchanged propafenone is excreted neither in the urine nor in the feces in amounts of more than 1 % the dose. The percentage of free unconjugated propafenone of the total deuterium content in the plasma was about 10 % 3 and 6 hours after application. Conjugates of hydroxylated derivatives of propafenone are present predominantly in the plasma.
The excretion of the metabolites takes place mainly by way of the feces, 53 % of the dose is excreted by this route within 48 hours. Urinary elimination accounted for 18.5 and 38 % of the dose within 48 hours in two humans.
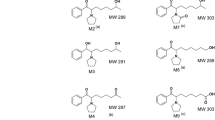
It was possible to elucidate the structure of 11 metabolites, accounting for more than 90 % of the dose administered. The major metabolites are conjugates of 5-hydroxypropafenone and hydroxy-methoxy-propafenone with glucuronic and sulphuric acid and propafenone glucuronide.
Furthermore, metabolic products of oxydative deamination pathway were identified, i.e. a glycol and a lactic acid derivative. C-C splitting yields a relatively large amount of 3-phenyl-propionic acid, while cleavage of the ether group leads to a phenolic product and is only of minor importance.
Similar content being viewed by others
References
Hege, H.G., Lietz, H. Weymann, J. (1984): Untersuchungen zum Metabolismus von Propafenon Arzneimittel-Forschung (Drug Research), in press.
Hege, H.G., Weymann, J. (1982): in Stable Isotopes, Proceedings of the 4th International Conference, Volume 11 of Analytical Chemistry Symposia Series. Ed.: Schmidt, H.L., Forstel, H., Heinzinger, K. p. 679–684. Elsevier Scientific Publishing Company, Amsterdam, Oxford, New York.
Barlow J.J., Kellie A.E. (1959): Biochem. J.: A quantitative method for the chromatographic separation of 17-Oxo Steroid Sulphates from 17-Oxo-Steroid-Glucuronides71, 86–91.
Brenner, K.S. (1978): Practical experience with a microwave plasma detector. Limits of measurement and examples of applications. J. Chromatogr.167, 365–380.
Riggs, C.E., Benjamin, R.S., Serpich, A.A., Bachur, N. (1977): Clin. Pharmacol./Therap.: Biliary disposition of adriamycin.22, 234–241.
Blanke, H., Aschbrenner, B., Karsch, K.R., Kreuzer, H. (1979): Dtsch. med. Wschr.: Plasmaspiegel-Wirkungs-Beziehung und Organverteilung von Propafenon104, 587–591.
Fenselau, C., Johnson, L.P. (1980): Drug Metab. Dispos.: Analysis of intact glucuronides by mass spectrometry and gas chromatography- mass spectrometry.8, 274–283.
Hoffmann, K.J., Arfwidsson, A., Borg, K.O. (1982): Drug Metab. Dispos.: The metabolic disposition of the selective β1 agonist prenalterol in mice, rat, dog and humans.10, 173–179.
Garteiz, D.A., Walle, T. (1972): J. Pharm. Sci.: Electron impact fragmentation studies of β-blocking drugs and their metbolites by GC-Mass Spectroscopy.61, 1728–1731.
Walle, T., Oatis, J.E., Walle, U.K., Knapp, D.R. (1982): Drug Metab. Dispos.: New ring-hydroxylated metabolites of propranolol.10, 122–127.
Walle, T., Conradi, E.C., Walle, U.K., Gaffney, T.E. (1978): Drug Metab. Dispos.: O-Methylated catechollike metabolites of propranolol in man.6, 481–487.
Bourne, G.R. «Progress in Drug Metabolism», (J.W. Bridges and L.F. Chasseaud, ed) (1981): p. 77, John Wiley & Sons Ltd.
Author information
Authors and Affiliations
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/BF03189625.
Rights and permissions
About this article
Cite this article
Hege, H.G., Hollmann, M., Kaumeier, S. et al. The metabolic fate of2H-labelled propafenone in man. European Journal of Drug Metabolism and Pharmacokinetics 9, 41–55 (1984). https://doi.org/10.1007/BF03189604
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03189604