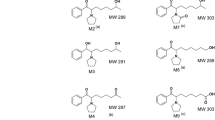
Urinary excretion of 9-(2-diethylaminoethyl)-2-phenylimidazo[1,2-a]benzimidazole dinitrate after intragastric administration at a dose of 10 mg/kg has been studied in laboratory rats. The main metabolite in blood serum of rats was determined after a single intravenous injection at a dose of 10 mg/kg. The metabolite was determined in urine samples using HPLC. Its structure was established using HPLC-MS. According to the results, the drug substance was excreted unchanged in small amounts (~0.01% of the administered dose) because it was metabolized. The main metabolite was found in rat plasma as early as 3 min after intravenous injection at a dose of 10 mg/kg.





Similar content being viewed by others
References
A. A. Spasov, V. I. Petrov, V. A. Anisimova, et al., RU Pat. 2,395,282 C2, Jul. 27, 2010; Byull. Izobret., No. 21 (2010).
D. O. Mitrakova, A. M. Kirilyuk, M. V. Chernikov, et al., Eksp. Klin. Farmakol., 84(11), 29 – 34 (2021).
D. O. Stupina, I. P. Remezova, and A. V. Morozov, In the Name of Life and Health: Proceedings of the 71st International Scientific-Practical Conference [in Russian], Pyatigorsk (2018).
D. O. Mitrakova, I. P. Remezova, and A. V. Morozov, Belikov Lectures: Proceedings of the IXth International Scientific-Practical Conference [in Russian], Pyatigorsk (2021).
D. O. Stupina, I. P. Remezova, and A. V. Morozov, Belikov Lectures: Proceedings of the VIIth All-Russian Scientific-Practical Conference [in Russian], Pyatigorsk (2019).
RF National Standard GOST R 33044-2014, Good Laboratory Practice Rules, enacted Aug. 1, 2015.
RF Ministry of Health and Social Development Order No. 199n of Apr. 1, 2016, On Approval of Good Laboratory Practice Rules.
A. N. Mironov (ed.), Handbook for Preclinical Drug Studies [in Russian], Part 1, Grif i K, Moscow (2012).
Acknowledgments
The chemical part of the article was prepared with the assistance of Southern Federal University with financial support from the RF Ministry of Science and Higher Education [State Task for Scientific Activity, Southern Federal University, 2020, Project FENW-2020-0031 (0852-2020-0031)].
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 56, No. 8, pp. 11 – 16, August, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mitrakova, D.O., Chernikov, M.V., Remezova, I.P. et al. Urinary Excretion Kinetics of 9-(2-Diethylaminoethyl)-2-Phenylimidazo[1,2-a]Benzimidazole Dinitrate and Determination of the Main Metabolite in Blood Serum of Laboratory Animals. Pharm Chem J 56, 1027–1032 (2022). https://doi.org/10.1007/s11094-022-02747-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02747-3