Abstract
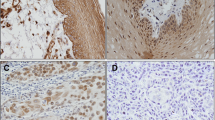
Cell-cell and cell-extracellular matrix interaction is crucial in tumor progression. Tight junction (TJ) proteins as occludin and claudins (CLDNs) play important role in this process together with several extracellular matrix components, as syndecan. Our previous work suggested significant changes in the expression of claudins even in the early stages of cervical carcinogenesis. The aim of our present work was to study the expression of occludin and syndecan-1, as compared to CLDNs, in early phases of cervical carcinogenesis. Paraffin sections of 50 samples were studied by immunohistochemistry, including cervical intraepithelial neoplasias (CIN-I-II-III), in situ carcinomas (CIS) and normal cervical samples. Occludin and CLDN-2 were found colocalized in the basal layer, while syndecan-1 and CLDN-1, -4 and -7 were coexpressed in the parabasal and intermedier layers in normal epithelia. Intensity of occludin staining decreased in CIN/CIS lesions, although it was more extended towards the upper epithelial layers with inverse relation with grades, as seen in the case of CLDN -2 expression. CLDN -1, -2, -4 and -7 were detected in the entire epithelium in CIN, showing decrease in CIS. The progression of CIN was associated with reduced syndecan-1 expression, in contrast to CLDN -1, -4 and -7 which increased toward CIS. The obtained data suggest that significant changes occur in the composition of cell adhesion complexes even in early stages of cervical carcinogenesis. The pattern of expression is characteristic for the alteration, the changes in the different components, however, are not parallel with each other.
Similar content being viewed by others
References
Acharya P, Beckel J, Ruiz WG, et al: Distribution of the tight junction proteins ZO-1, occludin, and claudin -4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol 287: F305-F318, 2004
Anttonen A, Kajanti M, Heikkila P, et al: Syndecan-1 expression has a prognostic significance in head and neck carcinoma. Br J Cancer 79: 558–564, 1999
Anttonen A, Heikkila P, Kajanti M, et al: High syndecan-1 expression is associated with favourable outcome in squamous cell lung carcinoma treated with radical surgery. Lung Cancer 32: 297–305, 2001
Bazer-Garner IB, Dilaz B, Sanderson RD andSmoller BR: Syndecan-1 expression is decreased with increasing aggressiveness of basal cell carcinoma. Am J Dermatopathol 22: 119–122, 2000
Billings SD, Walsh SV, Fisher C, et al: Aberrant expression of tight junction-related proteins ZO-1, claudin-1 and occludin in synovial sarcoma: an immunohistochemical study with ultrastructural correlation. Mod Pathol 17: 141–149, 2004
Chiba H, Gotoh T, Kojima T, et al: Hepatocyte nuclear factor (HNF)-4a triggers formation of functional tight junctions and establishment of polarized epithelial morphology in F9 embryonal carcinoma cells. Exp Cell Res 286: 288–297, 2003
Fujiya M, Watari J, Ashida T, et al: Reduced expression of syndecan1 affects metastatic potential and clinical outcome in patients with colorectal cancer. Jpn J Cancer Res 92: 1078–1081, 2001
Furuse M, Fujita K, Hiiragi T, et al: Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141: 1539–1550, 1998
Giroglou T, Florin L, Schäfer F, et al: Human papillomavirus infection requires cell surface heparan sulfate. J Virol 75: 1565–1570, 2001
Gumbiner BM: Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 84: 345–357, 1996
Hirohashi S, Kanai Y: Cell adhesion system and human cancer morphogenesis. Cancer Sci 94: 575–581, 2003
Hodivala KJ, Watt FM: Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J Cell Biol 124: 589–600, 1994
Hughes DE, Rebello G, Al-Nafussi A: Integrin expression in squamous neoplasia of the cervix. J Pathol 173: 97–104, 1994
Inki P, Stenback F, Grenman S, Jalkanen M: Immunohistochemical localization of syndecan in normal and pathological human uterine cervix. J Pathol 172: 349–355, 1994
Johnson LG: Applications of imaging techniques to studies of epithelial tight junctions. Adv Drug Deliver Rev 57: 111–121, 2005
Kominsky SL, Argani P, Korz D, et al: Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene 22: 2021–2033, 2003
Langbein L, Grund C, Kuhn C, et al: Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. Europ J Cell Biol 81: 419–435, 2002
Litvinov SV, van Uriel W, van Rhijn CM, et al: Expression of EpCAM in cervical squamous epithelia correlates with an increased proliferation and the disappearance of markers for terminal differentiation. Am J Pathol 148: 865–875, 1996
Mikami S, Ohashi K, Usui Y, et al: Loss of syndecan-1 and increased expression of heparanase in invasive esophageal carcinomas. Jpn J Cancer Res 92: 1062–1073, 2001
Miwa N, Furuse M, Tsukita S, et al: Involvement of claudin-1 in the beta-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res 12: 469–476, 2000
Morita K, Tsukita S, Miyachi Y: Tight junction-associated proteins (occludin, ZO-1, claudin-1, claudin-4) in squamous cell carcinoma and Bowen’s disease. Br J Dermatol 151: 328–334, 2004
Nakanishi K, Yoshioka N, Oka K, Hakura A: Reduction of syndecan-1 mRNA in cervical-carcinoma cells is involved with the 3′ untranslated region. Int J Cancer 80: 527–532, 1999
Nichols LS, Ashfaq R andIacobuzio-Donahue CA: Claudin 4 protein expression in primary and metastatic pancreatic cancer: support for use as a therapeutic target. Am J Clin Pathol 121: 226–230, 2004
Numa F, Hirabayashi K, Kawasaki K, et al: Syndecan-1 expression in cancer of the uterine cervix: association with lymph node metastasis. Int J Oncol 20: 39–43, 2002
Páska C, Bögi K, Szilák L, et al: Effect of formalin, acetone and RN Alater fixatives on tissue preservation and different size amplicons by real-time PCR from paraffin-embedded tissue. Diagn Mol Path 13: 234–240, 2004
Peppi M, Ghabriel MN: Tissue specific expression of the tight junction proteins claudins and occludin in the rat salivary glands. J Anat 205: 257–266, 2004
Rangel LB, Agarwal R, D’Souza T, etal: Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin Cancer Res 9: 2567–2575, 2003
Rintala J, Inki P, Klemi P, et al: Association of syndecan-1 with tumor grade and histology in primary invasive cervical carcinoma. Gynecol Oncol 75: 372–378, 1999
Schneeberger EE, Lynch RD: The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286: C1213-C1228, 2004
Shafti-Keramat S, Handisurya A, Kriehuber E, et al: Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J Virol 77: 13125–13135, 2003
Sobel G, Szabó I, Kiss A, et al: Increased expression of claudins in cervical squamous intraepithelial neoplasia and invasive carcinoma. Hum Pathol 36: 162–169, 2005
Tímár J, Lapis K, Dudás J, et al: Proteoglycans and tumor progression: Janus-faced molecules with contradictory functions in cancer. Semin Cancer Biol 12: 173–186, 2002
Tobioka H, Isomura H, Kokai Y, et al: Occludin expression decreases with the progression of human endometrial carcinoma. Hum Pathol 35: 159–164, 2004
Tobioka H, Tokunaga Y, Isomura H, et al: Expression of occludin, a tight-junction-associated protein, in human lung carcinomas. Virchows Arch 445: 472–476, 2004
Tőkés A-M, Kulka J, Paku S, et al: Claudin -1, -3 and -4 proteins and mRNA expression in benign and malignant breast lesions: a research study. Breast Cancer Res 7: R296-R305, 2005
Tsukita S, Furuse M, Itoh M: Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2: 285–293, 2001
Turksen K, Troy T-C: Barriers built on claudins. J Cell Sci 117: 2435–2447, 2004
Wiksten JP, Lundin J, Nordling S, et al: Epithelial and stromal syndecan-1 expression as predictor of outcome in patients with gastric cancer. Int J Cancer 95: 1–6, 2001
Woodworth CD, Waggoner S, Barnes W, et al: Human cervical and foreskin epithelial cells immortalized by human papillomavirus DNAs exhibit dysplastic differentiation in vivo. Cancer Res 50: 3709–3715, 1990
Yang Y, Yaccoby S, Liu W, et al: Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood 100: 610–617, 2002
Zheng JY, Yu D, Foroohar M, et al: Regulation of the expression of the prostate-specific antigen by claudin-7. J Membr Biol 194: 187–197, 2003
Author information
Authors and Affiliations
Additional information
This work was supported by grants NKFP-1A/0023/2002 from the Hungarian Ministry of Health, National Research Development Projects, OTKA T037838 from the National Science Research Foundation and ETT-077/2003 from the Hungarian Ministry of Health
Rights and permissions
About this article
Cite this article
Sobel, G., Szabó, I., Páska, C. et al. Changes of cell adhesion and extracellular matrix (ECM) components in cervical intraepithelial neoplasia. Pathol. Oncol. Res. 11, 26–31 (2005). https://doi.org/10.1007/BF03032402
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03032402