Abstract
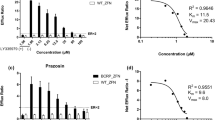
The purpose of this study was to investigate the utility of stably transfected MDCK-hPepT1 cells for identifying peptide transporter substrates in early drug discovery and compare the characteristics of this cell line with Caco-2 cells. MDCK-hPepT1, MDCK-mock, and Caco-2 cells grown to confluence on 24-well Transwell were used for this study. Expression levels of different transporter proteins (PepT1, PepT2, P-gp) in these cell lines were assessed by qRT-PCR. Permeability studies were conducted in parallel in all the cells with a diverse set of peptide substrates using the optimized experimental condition: 100 μM, apical pH 6.0, basolateral pH 7.4, 2 hr incubation at 37°C. Permeability studies were also conducted with classical P-gp substrates (tested in bi-directional mode) and paracellularly absorbed probes to investigate the differences between the cell lines. As expected, MDCK-hPepT1 cells express significantly higher level of PepT1 mRNA compared to both Caco-2 and MDCK-mock cells. Efflux transporter, P-gp, was expressed adequately in all the cell lines. Permeability studies demonstrated that classical peptide substrates had significantly higher permeability in stably transfected MDCK-hPepT1 cells compared to MDCK-mock and Caco-2 cells. The transfected MDCK-hPepT1 cells were qualitatively similar to Caco-2 cells with respect to functional P-gp efflux activity and paracellular pore activity. Stably transfected MDCK-hPepT1 cells have been demonstrated as a viable alternative to Caco-2 cells for estimating the human absorption potential of peptide transporter substrates. These cells behave similar to Caco-2 cells with regards to P-gp efflux and paracellular pore activity but demonstrate greater predictability of absorption values for classical peptide substrates (for which Caco-2 cells under-estimate oral absorption).
Similar content being viewed by others
References
Anderte, P., Rakhmanova, V., Woodford, K., Zerangue, N., and Sadee, W., Messenger RNA expression of transporter and ion channel genes in undifferentiated and differentiated Caco-2 cells compared to human intestines.Pharm. Res., 20, 1, 3–15 (2003).
Artursson, P. and Karlsson, J., Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelia (Caco-2) cells.Biochem. Biophys. Res. Comm., 175, 880–890 (1991).
Balimane, P. V., Chong, S., and Morrison, R. A., Current methodologies used for evaluation of intestinal permeability and absorption.J. Pharmacol. Toxicol. Methods., 44, 301- 312 (2000).
Balimane, P. V., Tamai, I., Guo, A., Nakanish, T., Takeo, K., Hideyuki, L., Frederick, H., Tsuji, A., and Sinko, P. J., Direct evidence for peptide transporter (PepT1)-mediated uptake of a nonpeptide prodrug, valacyclovir.Biochem. Biophys. Res. Commun., 250, 246–251 (1998).
Balimane, P. V., Patel, K., Marino, A., and Chong, S., Utility of 96 well Caco-2 cell system for increased throughput of P-gp screening in drug discovery.Eur. J. Pharm. Biopharm., 58, 99–105 (2004)
Balimane, P. V. and Sinko, P., Effect of ionization on the variable uptake of valacyclovirvia the human intestinal peptide transporter (hPepT1) in CHO cells.Biopharm. Drug. Dispos., 21, 165–174 (2000)
Balimane, P. V. and Chong, S., Cell culture-based models for intestinal permeability: a critique.Drug. Discov. Today., 10, 335–343 (2005).
Bretschneider, B., Brandsch, M., and Neubert, R., Intestinal transport of beta-lactam antibiotics: analysis of the affinity at the H+/peptide symporter (PEPT1), the uptake into Caco-2 cell monolayers and the transepithelial flux.Pharm. Res., 16, 55–61 (1999).
Cho, M., Thomson, D., Cramer, C., Vidmar, T., and Scieszka, J., The MDCK epithelial cell monolayer as a model cellular transport barrier.Pharm. Res., 6, 71–77 (1989)
Chong, S., Dando, S. A., Soucek, K. M., and Morrison, R. A.,In vitro permeability through caco-2 cells is not quantitatively predictive ofin vivo absorption for peptide-like drugs absorbedvia the dipeptide transporter system.Pharm. Res., 13, 120–123 (1996)
Chong, S., Dando, S. A., and Morrison, R. A., Evaluation of Biocoat intestinal epithelium differentiation environment (3- day cultured Caco-2 cells) as an absorption screening model with improved productivity.Pharm Res., 14, 1835–1837 (1997).
Chu, X. Y., Sanchez-Castano, G. P., Higaki, K., Oh, D. M., Hsu, C. P., and Amidon, G. L., Correlation between epithelial cell permeability of cephalexin and expression of intestinal oligopeptide transporter.J. Pharmacol. Exp. Ther., 299, 575- 582 (2001).
Collett, A., Sims, E., Walker, D., He, Y. L., Ayrton, J., Rowland, M., and Warhust, G., Comparison of HT29-18-C1 and Caco-2 cell lines as models for studying intestinal paracellular drug absorption.Pharm. Res., 13, 216–221 (1996).
Dantzig, A. H., Duckworth, D. C., and Tabas, L. B., Transport mechanisms responsible for the absorption of loracarbef, cefixime, and cefuroxime axetil into human intestinal Caco-2 cells.Biochim. Biophys. Acta., 1191, 7–13 (1994).
Daugherty, A. L. and Mrsny, R. J., Regulation of the intestinal epithelial paracellular barrier.Pharm. Sci. Technol. Today., 2, 281–287(1999).
Egan, W. J. and Lauri, G., Prediction of intestinal permeability.Adv. Drug. Deliv. Rev., 54, 273–289 (2002).
Ekins, S., Waller, C. L., Swaan, P. W., Cruciani, G., Wrighton, S. A., and Wikel, J. H., Progress in predicting human ADME parameters in silico.J. Pharmacol. Toxicol. Methods, 44, 251–272 (2000).
Faria, T. N., Timoszyk, J. K., Stouch, T., Vig, B. S., Landowski, C. P., Amidon, G. L., Weaver, C. D., Wall, D. A., and Smith, R. L., A Novel High Throughput PepT1 Transporter Assay Differentiates Between Substrates and Antagonists.Mol. Pharmaceutics., 1, 67–76 (2004).
Fei, Y. J., Ganapathy, V., and Leibach, F. H., Molecular and structural features of the proton-coupled oligopeptide transporter superfamily.Prog. Nucleic. Acid. Res. Mol. Biol., 58, 239–261 (1998).
Friedman, D. I. and Amidon, G. L., Intestinal absorption mechanism of dipeptide angiotensin converting enzyme inhibitors of the lysyl-proline type: lisinopril and SQ 29,852.J. Pharm. Sci., 78, 995–998 (1989)
Ganapathy, V. and Leibach, F. H., Peptide transporters.Curr Opin. Nephrol. Hypertens., 5, 395–400 (1996).
Guo, A., Hu, P., Balimane, P. V., Leibach, F. H., and Sinko, P. J., Interactions of a nonpeptidic drug, valacyclovir, with the human intestinal peptide transporter (hPEPT1) expressed in a mammalian cell line.J. Pharmacol. Exp. Ther., 289, 448- 454 (1999).
Han, H. K., Oh, D. M., and Amidon, G. L., Cellular uptake mechanism of amino acid ester prodrugs in Caco-2/hPEPT1 cells overexpressing a human peptide transporter.Pharm. Res., 15, 1382–1386 (1998)
Han, H., de Vrueh, R. L., Rhie, J. K., Covitz, K. M. Y., Smith, P. L., Lee, C. P., Oh, D. M., Sadee, W., and Amidon, G., 5’- Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter.Pharm. Res., 15, 1154–1159 (1998)
Herrera-Ruiz, D., Faria, T. N., Bhardwaj, R. K., Timoszyk, J., Gudmvndsson, O. S., Moench, P., Wall, D. A., Smith, R. L., and Knipp, G. T., A novel hPepT1 stably transfected cell line: establishing a correlation between expression and function.Mol. Pharmaceutics, 1, 136–144 (2004).
Hidalgo, I. J., Assessing the absorption of new Pharmaceuticals.Curr. Top. Med. Chem., 1, 385–401 (2001).
Ingels, F. M. and Augustijns, P. F., Biological, pharmaceutical, and analytical considerations with respect to the transport media used in the absorption screening system, Caco-2.J. Pharm. Sci., 92, 1545–1558 (2003)
Inui, K., Terada, T., Masuda, S., and Saito, H., Physiological and pharmacological implications of peptide transporters, PEPT1 and PEPT2.Nephrol Dial Transplant, 15 Suppl 6:11–13 (2000).
Irvine, J. D., Takahashi, L., Lockhart, K., Karen, C. J., Tolan, J. W., Selick, H. E., and Grove, J. R., MDCK (Madin-Darby canine kidney) cells: A tool for membrane permeability screening.J. Pharm. Sci., 88, 28–33 (1999).
Kansy, M., Senner, F., and Gubernator, K., Physicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes.J. Med. Chem., 41, 1007–1010 (1998).
Kennedy, D. J., Leibach, F. H., Ganapathy, V., and Thwaites, D. T., Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity.Pflugers. Arch., 445, 139–146 (2002).
Kerns, E., High throughput physicochemical profiling for drug discovery.J. Pharm. Sci., 90, 1838–1858 (2001).
Kottra, G., Stamfort, A., and Daniel, H., PEPT1 as a paradigm for membrane carriers that mediate electrogenic bidirectional transport of anionic, cationic, and neutral substrates.J. Biol. Chem., 277, 32683–32691 (2002).
Leveque, D. and Jehl, F., P-glycoprotein and pharmacokinetics.Anticancer. Res., 15, 331–336 (1995)
Li, H., Chung, S. J., and Shim, C. K., Characterization of the transport of uracil across Caco-2 and LLC-PK1 cell monolayers.Pharm. Res., 19, 1495–1501 (2002).
Lipinski, C. A., Lombardo, F., Dominy, B. W., and Feeney, P. J., Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings.Adv. Drug. Deliv. Rev., 46, 3–26 (2001).
Marino, A., Yarde, M., Patel, H., Chong, S., and Balimane, P., Validation of the 96 well Caco-2 cell culture model for high throughput permeability assessment of discovery compounds.Int. J. Pharm., 297, 235–241 (2005).
Meredith, D. and Boyd, C. A., Structure and function of eukaryotic peptide transporters.Cell Mol. Life Sci., 57, 754–778 (2000).
Opera, T. and Gottfries, J., Toward minimalistic modeling of oral drug absorption.J. Mol. Graph. Modeling, 17, 261–274 (1999).
Polli, J., Wring, S., Humphreys, J., Huang, L., Morgan, J. B., Webster, L. O., Serabjit, S., and Casette, S., Rational use ofin vitro P-gp assays in drug discovery.J. Pharmacol, and Exper. Therapeutics., 299, 620–628 (2001)
Putnam, W., Ramanathan, S., Pan, L., Takahashi, L., and Benet, L., Functional characterization of monocarboxylic acid, large neutral amino acid, bile acid and peptide transporters, and P-gp in MDCK and Caco-2 cells.J Pharm Sci., 91, 2622–2635 (2002).
Putnam, W. S., Pan, L., Tsutsui, K., Takahashi, L., and Benet, L. Z., Comparison of bidirectional cephalexin transport across MDCK and caco-2 cell monolayers: interactions with peptide transporters.Pharm. Res., 19, 27–33 (2002).
Rubio-Aliaga, I. and Daniel, H., Mammalian peptide transporters as targets for drug delivery.Trends. Pharmacol. Sci., 23, 434–440 (2002).
Sinko, P. J. and Balimane, P. V., Carrier-mediated intestinal absorption of valacyclovir, the L-valyl ester prodrug of acyclovir: 1. Interactions with peptides, organic anions and organic cations in rats.Biopharm. Drug. Dispos., 19, 209–217 (1998).
Swaan, P. W. and Tukker, J. J., Molecular determinants of recognition for the intestinal peptide carrier.J. Pharm. Sci., 86, 596–602 (1997).
Takeda, M., Babu, E., Narikawa, S., and Endou, H., Interaction of human organic anion transporters with various cephalo-sporin antibiotics.Eur. J. Pharmacol., 438, 137–142 (2002).
Tamai, I., Nakanishi, T., Hayashi, K., Terao, T., Sai, Y., Shiraga, T., Miyamoto, K. I., Takeda, E., Higashida, H., and Tsvji, A., he predominant contribution of oligopeptide transporter PepT1 to intestinal absorption of beta-lactam antibiotics in the rat small intestine.J. Pharm. Pharmacol., 49, 796–801 (1997).
Tang, F., Horie, K., and Borchardt, R., Are MDCK cells transfected with the human MDR1 gene a good model of the human intestinal mucosa.Pharm. Res., 19, 765–772 (2002).
Thwaites, D. T., Cavet, M., and Hirst, B. H., Simmons NL. Angiotensin-converting enzyme (ACE) inhibitor transport in human intestinal epithelial (Caco-2) cells.Br. J. Pharmacol., 114, 981–986 (1995).
Tsuji, A. and Tamai, I., Carrier-mediated intestinal transport of drugs.Pharm. Res., 13, 963–977 (1996).
Tsuji, A., P-glycoprotein-mediated efflux transport of anticancer drugs at the blood-brain barrier.Ther. Drug. Monit, 20, 588–590 (1998).
Walter, E., Kissel, T., and Amidon, G. L., The intestinal peptide carrier: a potential transport system for small peptide derived drugs.Adv. Drug Del. Rev, 20, 33–58 (1996).
Wang, H. P., Bair, C. H., and Huang, J. D., Uptake of cefadroxil derivatives in rat intestinal brush-border membrane vesicles.J. Pharm. Pharmacol., 44, 1027–1029 (1992).
Wu, C. Y. and Benet, L. Z., Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system.Pharm. Res., 22, 11–23 (2005).
Yang, C. Y., Dantzig, A. H., and Pidgeon, C., Intestinal peptide transport systems and oral drug availability.Pharm. Res., 16, 1331–1343(1999).
Zhao, Y. H., Abraham, M., Le, J., Hersey, A., Lusvambe, C. N., Beck, G., Sherborne, B., and Cooper, I., Rate-limited step of human oral absorption and QSAR studies.Pharm. Res., 19, 1446–1457 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balimane, P.V., Chong, S., Patel, K. et al. Peptide transporter substrate identification during permeability screening in drug discovery: Comparison of transfected MDCK-hPepT1 cells to caco-2 cells. Arch Pharm Res 30, 507–518 (2007). https://doi.org/10.1007/BF02980227
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02980227