Abstract
Purpose
We characterized three canine P-gp (cP-gp) deficient MDCKII cell lines. Their relevance for identifying efflux transporter substrates and predicting limitation of brain penetration were evaluated. In addition, we discuss how compound selection can be done in drug discovery by using these cell systems.
Method
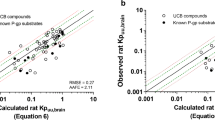
hMDR1, hBCRP-transfected, and non-transfected MDCKII ZFN cells (all with knock-down of endogenous cP-gp) were used for measuring permeability and efflux ratios for substrates. The compounds were also tested in MDR1_Caco-2 and BCRP_Caco-2, each with a double knock-out of BCRP/MRP2 or MDR1/MRP2 transporters respectively. Efflux results were compared between the MDCK and Caco-2 models. Furthermore, in vitro MDR1_ZFN efflux data were correlated with in vivo unbound drug brain-to-plasma partition coefficient (Kp,uu).
Results
MDR1 and BCRP substrates are correctly classified and robust transporter affinities with control substrates are shown. Cell passage mildly influenced mRNA levels of transfected transporters, but the transporter activity was proven stable for several years. The MDCK and Caco-2 models were in high consensus classifying same efflux substrates. Approx. 80% of enlisted substances were correctly predicted with the MDR1_ZFN model for brain penetration.
Conclusion
cP-gp deficient MDCKII ZFN models are reliable tools to identify MDR1 and BCRP substrates and useful for predicting efflux liability for brain penetration.






Similar content being viewed by others
Abbreviations
- ABC:
-
ATP-binding cassette
- API:
-
Active pharmaceutical ingredient
- BCRP:
-
Breast cancer resistance protein
- CNS:
-
Central nervous system
- cP-gp:
-
Canine P-glycoprotein
- Cpm:
-
Count per minute
- CT :
-
Cycle threshold
- DMEM:
-
Dulbecco’s modified eagle medium
- DMSO:
-
Dimethyl sulfoxide
- DPBS:
-
Dulbecco’s phosphate buffered saline
- EDTA:
-
Ethylenediaminetetraacetic acid
- EMA:
-
European medicines agency
- ER:
-
Efflux ratio
- FBS:
-
Fetal bovine serum
- FDA:
-
U.S. Food and drug administration
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- HBSS:
-
Hank’s balanced salt solution
- MDCK:
-
Madin-Darby canine kidney
- MDR:
-
Multidrug resistance
- mRNA:
-
Messenger RNA
- MRP:
-
Multidrug resistance associated protein
- NEA:
-
Non-essential amino acids
- NER:
-
Net efflux ratio
- OATP:
-
Organic anion-transporting polypeptide
- OCT:
-
Organic cation transporter
- Papp :
-
Apparent permeability coefficient
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- P-gp:
-
P-glycoprotein
- WT:
-
Wild type
- ZFN:
-
Zinc finger nuclease
References
Guidance for Industry: In Vitro Metabolism and Transporter Mediated Drug-Drug Interaction Studies. 2017. https://wwwfdagov/media/108130/download.
Hellinger É, Veszelka S, Tóth AE, Walter F, Kittel Á, Bakk ML, et al. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VB-Caco-2) cell-based surrogate blood–brain barrier penetration models. Eur J Pharm Biopharm. 2012;82(2):340–51.
Garberg P, Ball M, Borg N, Cecchelli R, Fenart L, Hurst R, et al. In vitro models for the blood–brain barrier. Toxicol in Vitro. 2005;19(3):299–334.
Sai Y, Tsuji A. Transporter-mediated drug delivery: recent progress and experimental approaches. Drug Discov Today. 2004;9(16):712–20.
Sampson KE, Brinker A, Pratt J, Venkatraman N, Xiao Y, Blasberg J, et al. Zinc finger nuclease–mediated gene knockout results in loss of transport activity for P-glycoprotein, BCRP, and MRP2 in Caco-2 cells. Drug Metab Dispos. 2015;43(2):199–207.
Dukes JD, Whitley P, Chalmers AD. The MDCK variety pack: choosing the right strain. BMC Cell Biol. 2011;12:43.
Kikuchi R, de Morais SM, Kalvass JC. In vitro P-glycoprotein efflux ratio can predict the in vivo brain penetration regardless of biopharmaceutics drug disposition classification system class. Drug Metab Dispos. 2013;41(12):2012–7.
Feng B, West M, Patel NC, Wager T, Hou X, Johnson J, et al. Validation of human MDR1-MDCK and BCRP-MDCK cell lines to improve the prediction of brain penetration. J Pharm Sci. 2019;108:2476–83.
Feng B, Doran AC, Di L, West MA, Osgood SM, Mancuso JY, et al. Prediction of human brain penetration of P-glycoprotein and breast cancer resistance protein substrates using in vitro transporter studies and animal models. J Pharm Sci. 2018;107(8):2225–35.
Sato S, Tohyama K, Kosugi Y. Investigation of MDR1-overexpressing cell lines to derive a quantitative prediction approach for brain disposition using in vitro efflux activities. Eur J Pharm Sci. 2019;105119
Gartzke D, Delzer J, Laplanche L, Uchida Y, Hoshi Y, Tachikawa M, et al. Genomic knockout of endogenous canine P-glycoprotein in wild-type, human P-glycoprotein and human BCRP transfected MDCKII cell lines by zinc finger nucleases. Pharm Res. 2015;32(6):2060–71.
Simoff I, Karlgren M, Backlund M, Lindström A-C, Gaugaz FZ, Matsson P, et al. Complete knockout of endogenous Mdr1 (Abcb1) in MDCK cells by CRISPR-Cas9. J Pharm Sci. 2016;105(2):1017–21.
Karlgren M, Simoff I, Backlund M, Wegler C, Keiser M, Handin N, et al. A CRISPR-Cas9 generated MDCK cell line expressing human MDR1 without endogenous canine MDR1 (cABCB1): an improved tool for drug efflux studies. J Pharm Sci. 2017;106(9):2909–13.
Chen EC, Broccatelli F, Plise E, Chen B, Liu L, Cheong J, et al. Evaluating the utility of canine Mdr1 knockout Madin-Darby canine kidney I cells in permeability screening and efflux substrate determination. Mol Pharm. 2018;15(11):5103–13.
Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T. P-glycoprotein-mediated transcellular transport of MDR-reversing agents. FEBS Lett. 1993;324(1):99–102.
Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, et al. Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001;299(2):620–8.
Haslam IS, Jones K, Coleman T, Simmons N. Induction of P-glycoprotein expression and function in human intestinal epithelial cells (T84). Biochem Pharmacol. 2008;76(7):850–61.
Wang Q, Strab R, Kardos P, Ferguson C, Li J, Owen A, et al. Application and limitation of inhibitors in drug–transporter interactions studies. Int J Pharm. 2008;356(1–2):12–8.
Sane R, Agarwal S, Mittapalli RK, Elmquist WF. Saturable active efflux by p-glycoprotein and breast cancer resistance protein at the blood-brain barrier leads to nonlinear distribution of elacridar to the central nervous system. J Pharmacol Exp Ther. 2013;345(1):111–24.
Wang Z, Pal D, Patel A, Kwatra D, Mitra AK. Influence of overexpression of efflux proteins on the function and gene expression of endogenous peptide transporters in MDR-transfected MDCKII cell lines. Int J Pharm. 2013;441(1–2):40–9.
Volpe DA. Variability in Caco-2 and MDCK cell-based intestinal permeability assays. J Pharm Sci. 2008;97(2):712–25.
Heikkinen AT, Korjamo T, Lepikkö V, Mönkkönen J. Effects of experimental setup on the apparent concentration dependency of active efflux transport in in vitro cell permeation experiments. Mol Pharm. 2010;7(2):605–17.
Wright JA, Haslam IS, Coleman T, Simmons NL. Breast cancer resistance protein BCRP (ABCG2)-mediated transepithelial nitrofurantoin secretion and its regulation in human intestinal epithelial (Caco-2) layers. Eur J Pharmacol. 2011;672(1–3):70–6.
Cassio D. Long term culture of MDCK strains alters chromosome content. BMC Res Notes. 2013;6(1):162.
Lewin B. Genes IV. Am J Phys Anthropol. New York: Oxford University Press; 1990.
Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991;175(3):880–5.
Lennernäs H, Palm K, Fagerholm U, Artursson P. Comparison between active and passive drug transport in human intestinal epithelial (Caco-2) cells in vitro and human jejunum in vivo. Int J Pharm. 1996;127(1):103–7.
Larregieu CA, Benet LZ. Drug discovery and regulatory considerations for improving in silico and in vitro predictions that use Caco-2 as a surrogate for human intestinal permeability measurements. AAPS J. 2013;15(2):483–97.
Li J, Volpe DA, Wang Y, Zhang W, Bode C, Owen A, et al. Use of transporter knockdown Caco-2 cells to investigate the in vitro efflux of statin drugs. Drug Metab Dispos. 2011;39(7):1196–202.
Huang L, Wang Y, Grimm S. ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos. 2006;34(5):738–42.
Li J, Wang Y, Zhang W, Huang Y, Hein K, Hidalgo IJ. The role of a basolateral transporter in rosuvastatin transport and its interplay with apical breast cancer resistance protein in polarized cell monolayer systems. Drug Metab Dispos. 2012;40(11):2102–8.
Hirohashi T, Suzuki H, Chu X-Y, Tamai I, Tsuji A, Sugiyama Y. Function and expression of multidrug resistance-associated protein family in human colon adenocarcinoma cells (Caco-2). J Pharmacol Exp Ther. 2000;292(1):265–70.
Gartzke D, Fricker G. Establishment of optimized MDCK cell lines for reliable efflux transport studies. J Pharm Sci. 2014;103(4):1298–304.
Schinkel AH, Wagenaar E, van Deemter L, Mol C, Borst P. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995;96(4):1698–705.
Van Asperen J, Schinkel AH, Beijnen JH, Nooijen WJ, Borst P, van Tellingen O. Altered pharmacokinetics of vinblastine in Mdr1a P-glycoprotein-deficient mice. JNCI: J Natl Cancer Inst. 1996;88(14):994–9.
Evans DC, O’Connor D, Lake BG, Evers R, Allen C, Hargreaves R. Eletriptan metabolism by human hepatic CYP450 enzymes and transport by human P-glycoprotein. Drug Metab Dispos. 2003;31(7):861–9.
Schinkel A, Smit J, Van Tellingen M, Beijnen J, Wagenaar E, Van Deemter L, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77(4):491–502.
Kemper EM, Cleypool C, Boogerd W, Beijnen JH, van Tellingen O. The influence of the P-glycoprotein inhibitor zosuquidar trihydrochloride (LY335979) on the brain penetration of paclitaxel in mice. Cancer Chemother Pharmacol. 2004;53(2):173–8.
Kusuhara H, Suzuki H, Terasaki T, Kakee A, Lemaire M, Sugiyama Y. P-glycoprotein mediates the efflux of quinidine across the blood-brain barrier. J Pharmacol Exp Ther. 1997;283(2):574–80.
Hendrikse N, Schinkel A, De Vries E, Fluks E, Van der Graaf W, Willemsen A, et al. Complete in vivo reversal of P-glycoprotein pump function in the blood-brain barrier visualized with positron emission tomography. Br J Pharmacol. 1998;124(7):1413–8.
Pereira JNDS, Tadjerpisheh S, Abed MA, Saadatmand AR, Weksler B, Romero IA, et al. The poorly membrane permeable antipsychotic drugs amisulpride and sulpiride are substrates of the organic cation transporters from the SLC22 family. AAPS J. 2014;16(6):1247–58.
Wang J-S, Ruan Y, Taylor RM, Donovan JL, Markowitz JS, DeVane CL. The brain entry of risperidone and 9-hydroxyrisperidone is greatly limited by P-glycoprotein. Int J Neuropsychopharmacol. 2004;7(4):415–9.
Acknowledgement
The authors would like to thank Juliane Hoeckels-Messemer, Sylvia Hellwig and Patricia Muschong of AbbVie for their technical support.
Disclosures
Dong Ye, Manuel Weinheimer, Loic Laplanche, Mario Mezler are employees of AbbVie and may own AbbVie stock. Zhizhou Fang was an employee of AbbVie at the time when he contributed to the work being discussed, and he currently works at Merck Healthcare KGaA, Germany. Anna Harder was a master student of AbbVie for this work at the time and currently works at Braun Melsungen AG, Germany. The design, study conduct, and financial support for this research was provided by AbbVie. AbbVie participated in the interpretation of data, review, writing and approval of the publication. All authors declare no competing financial interest in this work.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Dong Ye, Anna Harder, Zhizhou Fang. The first draft of the manuscript was written by Dong Ye and all authors commented on previous versions of the manuscript. All authors actively contributed to the final text, read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ye, D., Harder, A., Fang, Z. et al. Characterization and Validation of Canine P-Glycoprotein-Deficient MDCK II Cell Lines for Efflux Substrate Screening. Pharm Res 37, 194 (2020). https://doi.org/10.1007/s11095-020-02895-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02895-9