Abstract
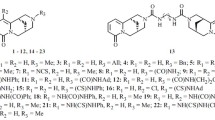
6-Arylamino-7-halo-5,8-quinolinediones (4a-4k, 5a-5b) were tested forin vitro cytotoxicity against human solid tumor cell lines such as A 549 (non-small cell lung), SK-OV-3 (ovarian), SK-MEL-2 (melanoma), HCT-15 (colon) and XF 498 (CNS) by SRB assay. The arylamino-7-chloro-5,8-quinolinediones4 were also evaluated for cyclin-dependent kinase (CDK2 and CDK4) inhibitory effect. Among them, the 5,8-quinolinediones4a and5a with 7-(4-fluorophenyl)amino group were found to be potent cytotoxic against HCT 15, SKOV-3 and XF 498, and the compounds4f and4i showed inhibitory activities for the CDK4.
Similar content being viewed by others
References
Boger, D. L., Yasuda, M., Mitscher, L. A., Drake, S. D., Kitos, P. A. and Thomson, S. C., Streptonigrin and lavandamycin partial structure. A probe for the minimum potent pharmacophore of streptonigrin, lavandamycin and synthetic quinoline-5,8-diones.J. Med. Chem., 30, 1918–1928 (1987).
Collins, K., Jacks, T. and Pavletich, N. P., The cell cycle and cancer.Proc. Natl. Acad. Sci. USA, 94 2776–2778 (1997).
Dunphy, W. G.,Methods in Enzymology; Cell Cycle Control. Academic Press, San Diego, pp 1–205, 1997; Phelps, D. E. and Xiong, Y. Assay for activity of mammalian cyclin D-dependent kinases CDK4 and CDK6, 194–205; Meijer, L. and Kim, S-.H. Chemical inhibitors of cyclin-dependent kinases, 113–128.
Jeschke, P., Linder, W., Mueller, N. Harder, A. and Mencke, N., New 6(7)amino-substituted-5,8-quinolinediones to combat endoparasites.Eur. Patent., Appl. EP 519, 290 (Cl. C07D215/38) (1992).
Lown, J. W. and Sim, S. K., Studies related to antitumor antibiotics. Synthesis of streptonigrin analogues and their single strand scission of DNA.Can. J. Chem., 54, 2563–2572 (1976).
Rao, K. V. and Beach, J. W., Streptonigrin and related compounds 5. Synthesis and evaluation of some isoquinolin analogues.J. Med. Chem., 34, 1871–1879 (1991).
Rao, K. V. and Rock, C. P., Streptonigrin and related compounds. 6. Synthesis and activity of some quinoxaline analogues.J. Heterocycl. Chem., 33, 447–458 (1996).
Ryu, C. K. and Kim, H. J., The synthesis of 6-(N-arylamino)-7-chloro-5,8-quinolinedione derivatives for evaluation of antifungal activities.Arch. Pharm. Res., 17, 139–144 (1994).
Ryu, C. K., Lee, I. K., Jung, S. H. and Lee, C. O., Synthesis and cytotoxic activities 6-chloro-7-arylamino-5,8-isoquinolinediones.Bioorg. Med. Chem. Lett., 9(8), 1075–1080 (1999a).
Ryu, C. K., Jung, S. H., Lee, J. A., Kim, H. J., Lee, S. H. and Chung, J. H., 6-Arylamino-5,8-quinolinediones and 7-arylamino-5,8-isoquinolinediones as inhibitors of endothelium-dependent vasorelaxation.Bioorg. Med. Chem. Lett., 9(17), 2466–2472 (1999b).
Sausville, E. A., Zahararevitz, D., Gussio, R., Meijer, L., Louarn-Leost, M., Kunick, C., Schultz, R., Lahusen, T., Headlee, D., Stinson, S., Arbuck, S. G. and Senderowicz, A., Cyclin-dependent kinases: Initial approaches to exploit a novel therapeutic target.Pharmacol. Ther., 82 285–292 (1999).
Schellhammer, C. W. and Petersen, S., Ueber Derivatives des 5,8-Chinolinchinone.Ann. der Chem., 624, 108–119 (1959).
Shaikh, I. A., Johnson, F. and Grollman, A. P., Structure-activity relationship among simple bicyclic analogues. Rate dependent of DNA degradation on quinone reduction potential.J. Med. Chem., 29, 1329–1340 (1986).
Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., Warren, T. W., Bokesch, H., Kenney, S. and Boyd, M. R., New colorimetric cytotoxicity assay for anticancer-drug screening.J. Natl. Cancer Inst., 82, 1107–1112 (1990).
Webster, K. R., The therapeutic potential of targeting the cell cycle.Exp. Opin. Invest. Drugs, 7(6), 865–887, (1998).
Yasuda, M. and Boger, D. L., Streptonigrin and lavandamycin partial structure. A probe for the minimum potent pharmacophore of the natural occurring antitumor-anitibiotics.J. Heterocycl. Chem., 24, 1253–1259 (1987).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryu, CK., Kang, HY., Yi, YJ. et al. Cytotoxic activities of 6-arylamino-7-halo-5,8-quinolinediones against human tumor cell lines. Arch Pharm Res 23, 42–45 (2000). https://doi.org/10.1007/BF02976464
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02976464