Abstract
Background
Adropin, a unique peptide hormone, has been associated with the regulation of several physiological processes, including glucose homeostasis, fatty acid metabolism, and neovascularization. However, its possible role in ovarian function is not understood. Our objective was to examine the expression of adropin and its putative receptor, GPR19, in the ovaries of mice at various phases of the estrous cycle.
Methods
Immunohistochemistry and western blot analysis were performed to explore the localization and changes in expression of adropin and GPR19 in the ovaries during different phases of the estrous cycle in mice. Hormonal assays were performed with ELISA. An in vitro study was performed to examine the direct effect of adropin (10, 100 ng/ml) on ovarian function.
Results
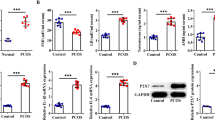
A western blot study showed that adropin and GPR19 proteins were maximum during the estrus phase of the estrous cycle. Interestingly, adropin and GPR19 displayed intense immunoreactivity in granulosa cells of large antral follicles and corpus luteum. This suggested the possible involvement of adropin in corpus luteum formation. Adropin treatment stimulated progesterone synthesis by increasing GPR19, StAR, CYP11A1, and 3β-HSD expressions, while it decreased estrogen synthesis by inhibiting 17β-HSD and aromatase protein expressions. Moreover, adropin treatment upregulated the cell cycle arrest-CDK inhibitor 1B (p27kip1), pERK1/2, and angiogenic protein (EG VEGF) that are involved in the process of luteinization.
Conclusions
Adropin GPR19 signaling promotes the synthesis of progesterone and upregulates the expression of p27kip1, EG VEGF, and erk1/2, resulting in cell cycle arrest and neovascularization, which ultimately leads to corpus luteum formation.








Similar content being viewed by others
References
de Oliveira dos Santos AR, de Oliveira Zanuso B, VFB M, Barbalho SM, Santos Bueno PC, UAP F et al (2021) Adipokines, myokines, and hepatokines: crosstalk and metabolic repercussions. Int J Mol Sci 22(5):2639. https://doi.org/10.3390/ijms22052639
Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V et al (2007) Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab 5(6):415–425. https://doi.org/10.1016/j.cmet.2007.05.003
Owen BM, Bookout AL, Ding X, Lin VY, Atkin SD, Gautron L et al (2013) FGF21 contributes to neuroendocrine control of female reproduction. Nat Med 19(9):1153–1156. https://doi.org/10.1038/nm.3250
Dietzel E, Wessling J, Floehr J, Schäfer C, Ensslen S, Denecke B et al (2013) Fetuin-B, a liver-derived plasma protein is essential for fertilization. Dev Cell 25(1):106–112. https://doi.org/10.1016/j.devcel.2013.03.001
Dietzel E, Floehr J, Jahnen-Dechent W (2016) The biological role of fetuin-B in female reproduction. Ann Reprod Med Treat 1(1):1003
Hammond GL (2011) Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod 85(3):431–441. https://doi.org/10.1095/biolreprod.111.092593
Kumar KG, Trevaskis JL, Lam DD, Sutton GM, Koza RA, Chouljenko VN et al (2008) Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab 8(6):468–481. https://doi.org/10.1016/j.cmet.2008.10.011
Aydin S, Kuloglu T, Aydin S, Eren MN, Yilmaz M, Kalayci M et al (2013) Expression of adropin in rat brain, cerebellum, kidneys, heart, liver, and pancreas in streptozotocin-induced diabetes. Mol Cell Biochem 380(1-2):73–81. https://doi.org/10.1007/s11010-013-1660-4
Stein LM, Yosten GL, Samson WK (2016) Adropin acts in brain to inhibit water drinking: potential interaction with the orphan G protein-coupled receptor, GPR19. Am J Physiol Regul Integr Comp Physiol 310(6):R476–R480. https://doi.org/10.1152/ajpregu.00511.2015
Rao A (1864) Herr DR (2017) G protein-coupled receptor GPR19 regulates E-cadherin expression and invasion of breast cancer cells. Biochim Biophys Acta Mol Cell Res 7:1318–1327. https://doi.org/10.1016/j.bbamcr.2017.05.001
Wong CM, Wang Y, Lee JTH, Huang Z, Wu D, Xu A et al (2014) Adropin is a brain membrane-bound protein regulating physical activity via the NB-3/Notch signaling pathway in mice. J Biol Chem 289(37):25976–25986. https://doi.org/10.1074/jbc.M114.576058
Ganesh Kumar K, Zhang J, Gao S, Rossi J, McGuinness OP, Halem HH et al (2012) Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity (Silver Spring) 20(7):1394–1402
Ghoshal S, Stevens JR, Billon C, Girardet C, Sitaula S, Leon AS et al (2018) Adropin: an endocrine link between the biological clock and cholesterol homeostasis. Mol Metab 8:51–64. https://doi.org/10.1016/j.molmet.2017.12.002
Gao S, McMillan RP, Zhu Q, Lopaschuk GD, Hulver MW, Butler AA (2015) Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Mol Metab 4(4):310–324. https://doi.org/10.1016/j.molmet.2015.01.005
Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta M et al (2010) Adropin is a novel regulator of endothelial function. Circulation 122((11_suppl_1)):S185–S192. https://doi.org/10.1161/CIRCULATIONAHA.109.931782
Celik E, Yilmaz E, Celik O, Ulas M, Turkcuoglu I, Karaer A et al (2013) Maternal and fetal adropin levels in gestational diabetes mellitus. J Perinat Med 41(4):375–380. https://doi.org/10.1515/jpm-2012-0227
Chen X, Xue H, Fang W, Chen K, Chen S, Yang W et al (2019) Adropin protects against liver injury in nonalcoholic steatohepatitis via the Nrf2 mediated antioxidant capacity. Redox Biol 21:101068. https://doi.org/10.1016/j.redox.2018.101068
Yu HY, Zhao P, Wu MC, Liu J, Yin W (2014) Serum adropin levels are decreased in patients with acute myocardial infarction. Regul Pept 190-191:46–49. https://doi.org/10.1016/j.regpep.2014.04.001
Yildirim B, Celik O, Aydin S (2014) Adropin: a key component and potential gatekeeper of metabolic disturbances in policystic ovarian syndrome. Clin Exp Obstet Gynecol 41(3):310–312. https://doi.org/10.12891/ceog16522014
Nergiz S, Altinkaya SO, Kurt Ömürlü İ, Yuksel H, Küçük M, Demircan Sezer S (2015) Circulating adropin levels in patients with endometrium cancer. Gynecol Endocrinol 31(9):730–735. https://doi.org/10.3109/09513590.2015.1065480
Celik A, Balin M, Kobat MA, Erdem K, Baydas A, Bulut M et al (2013) Deficiency of a new protein associated with cardiac syndrome X; called adropin. Cardiovasc Ther 31(3):174–178. https://doi.org/10.1111/1755-5922.12025
Sato K, Yamashita T, Shirai R, Shibata K, Okano T, Yamaguchi M et al (2018) Adropin contributes to anti-atherosclerosis by suppressing monocyte-endothelial cell adhesion and smooth muscle cell proliferation. Int J Mol Sci 19(5):1293. https://doi.org/10.3390/ijms19051293
Sen T, Saha P, Gupta R, Foley LM, Jiang T, Abakumova OS et al (2020) Aberrant ER stress induced neuronal-IFNβ elicits white matter injury due to microglial activation and T-cell infiltration after TBI. J Neurosci 40(2):424–446. https://doi.org/10.1523/JNEUROSCI.0718-19.2019
Maurya S, Singh A (2022) Asprosin modulates testicular functions during ageing in mice. Gen Comp Endocrinol 323-324:114036. https://doi.org/10.1016/j.ygcen.2022.114036
Singh A, Krishna A (2012) Localization of adiponectin and its receptor and its possible roles in the ovary of a vespertilionid bat Scotophilus heathi. Gen Comp Endocrinol 176(2):240–251. https://doi.org/10.1016/j.ygcen.2012.01.020
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem 72(1-2):248–254
Khajehnasiri N, Dehkordi MB, Amini-Khoei H, Mohammadabadi MSM, Sadeghian R (2021) Effect of exercise intensity and duration on the levels of stress hormones and hypothalamic-pituitary–gonadal axis in adult male rats: an experimental study. Hormones 20(3):483–490
Fitz TA, Mayan MH, Sawyer HR, Niswender GD (1982) Characterization of two steroidogenic cell types in the ovine corpus luteum. Biol Reprod 27(3):703–711. https://doi.org/10.1095/biolreprod27.3.703
Meyer GT, McGeachie JK (1988) Angiogenesis in the developing corpus luteum of pregnant rats: a stereologic and autoradiographic study. Anat Rec 222(1):18–25. https://doi.org/10.1002/ar.1092220105
Jablonka-Shariff A, Grazul-Bilska AT, Redmer DA, Reynolds LP (1993) Growth and cellular proliferation of ovine corpora lutea throughout the estrous cycle. Endocrinology 133(4):1871–1879. https://doi.org/10.1210/endo.133.4.8404629
Zheng J, Fricke PM, Reynolds LP, Redmer DA (1994) Evaluation of growth, cell proliferation, and cell death in bovine corpora lutea throughout the estrous cycle. Biol Reprod 51(4):623–632
Dickson SE, Fraser HM (2000) Inhibition of early luteal angiogenesis by gonadotropin-releasing hormone antagonist treatment in the primate. J Clin Endocrinol Metab 85(6):2339–2344. https://doi.org/10.1210/jcem.85.6.6621
Rodger FE, Young FM, Fraser HM, Illingworth PJ (1997) Endothelial cell proliferation follows the mid-cycle luteinizing hormone surge, but not human chorionic gonadotrophin rescue, in the human corpus luteum. Hum Reprod 12(8):1723–1729. https://doi.org/10.1093/humrep/12.8.1723
Gaytán F, Morales C, García-Pardo L, Reymundo C, Bellido C, Sánchez-Criado JE (1998) Macrophages, cell proliferation, and cell death in the human menstrual corpus luteum. Biol Reprod 59(2):417–425. https://doi.org/10.1095/biolreprod59.2.417
Devoto L, Kohen P, Muñoz A, Strauss JF III (2009) Human corpus luteum physiology and the luteal-phase dysfunction associated with ovarian stimulation. Reprod Biomed Online 18(Suppl 2):19–24. https://doi.org/10.1016/s1472-6483(10)60444-0
Lu E, Li C, Wang J, Zhang C (2019) Inflammation and angiogenesis in the corpus luteum. J Obstet Gynaecol Res 45(10):1967–1974. https://doi.org/10.1111/jog.14076
Fraser HM, Wulff C (2003) Angiogenesis in the corpus luteum. Reprod Biol Endocrinol 1(1):88. https://doi.org/10.1186/1477-7827-1-88
Wiltbank MC, Dysko RC, Gallagher KP, Keyes PL (1988) Relationship between blood flow and steroidogenesis in the rabbit corpus luteum. J Reprod Fertil 84(2):513–520. https://doi.org/10.1530/jrf.0.0840513
Niswender GD, Moore RT, Akbar AM, Nett TM, Diekman MA (1975) Flow of blood to the ovaries of ewes throughout the estrous cycle. Biol Reprod 13(4):381–388. https://doi.org/10.1095/biolreprod13.4.381
Ford SP, Christenson RK, Chenault JR (1979) Patterns of blood flow to the uterus and ovaries of ewes during the period of luteal regression. J Anim Sci 49(6):1510–1516. https://doi.org/10.2527/jas1979.4961510x
Janson PO, Damber JE, Axén C (1981) Luteal blood flow and progesterone secretion in pseudopregnant rabbits. J Reprod Fertil 63(2):491–497. https://doi.org/10.1530/jrf.0.0630491
Magness RR, Christenson RK, Ford SP (1983) Ovarian blood flow throughout the estrous cycle and early pregnancy in sows. Biol Reprod 28(5):1090–1096. https://doi.org/10.1095/biolreprod28.5.1090
Green C, Chatterjee R, McGarrigle HH, Ahmed F, Thomas NS (2000) p107 is active in the nucleolus in non-dividing human granulosa lutein cells. J Mol Endocrinol 25(3):275–286. https://doi.org/10.1677/jme.0.0250275
Jirawatnotai S, Moons DS, Stocco CO, Franks R, Hales DB, Gibori G, Kiyokawa H (2003) The cyclin-dependent kinase inhibitors p27Kip1 and p21Cip1 cooperate to restrict proliferative life span in differentiating ovarian cells. J Biol Chem 278(19):17021–17027. https://doi.org/10.1074/jbc.M301206200
Samson M, Peale FV Jr, Frantz G, Rioux-Leclercq N, Rajpert-De Meyts E, Ferrara N (2004) Human endocrine gland-derived vascular endothelial growth factor: expression early in development and in Leydig cell tumors suggests roles in normal and pathological testis angiogenesis. J Clin Endocrinol Metab 89(8):4078–4088. https://doi.org/10.1210/jc.2003-032024
Kisliouk T, Levy N, Hurwitz A, Meidan R (2003) Presence and regulation of endocrine gland vascular endothelial growth factor/prokineticin-1 and its receptors in ovarian cells. J Clin Endocrinol Metab 88(8):3700–3707. https://doi.org/10.1210/jc.2003-030492
Hoffmann P, Feige JJ, Alfaidy N (2007) Placental expression of EG VEGF and its receptors PKR1 (prokineticin receptor-1) and PKR2 throughout mouse gestation. Placenta 28(10):1049–1058. https://doi.org/10.1016/j.placenta.2007.03.008
Heck D, Wortmann S, Kraus L, Ronchi CL, Sinnott RO, Fassnacht M et al (2015) Role of endocrine gland-derived vascular endothelial growth factor (EG VEGF) and its receptors in adrenocortical tumors. Horm Cancer 6(5-6):225–236. https://doi.org/10.1007/s12672-015-0236-z
Brouillet S, Hoffmann P, Benharouga M, Salomon A, Schaal JP, Feige JJ et al (2010) Molecular characterization of EG-VEGF-mediated angiogenesis: differential effects on microvascular and macrovascular endothelial cells. Mol Biol Cell 21(16):2832–2843. https://doi.org/10.1091/mbc.E10-01-0059
Lin R, LeCouter J, Kowalski J, Ferrara N (2002) Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. J Biol Chem 277(10):8724–8729
Stocco C, Telleria C, Gibori G (2007) The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28(1):117–149. https://doi.org/10.1210/er.2006-0022
Topuz M, Celik A, Aslantas T, Demir AK, Aydin S, Aydin S (2013) Plasma adropin levels predict endothelial dysfunction like flow-mediated dilatation in patients with type 2 diabetes mellitus. J Investig Med 61(8):1161–1164. https://doi.org/10.2310/JIM.0000000000000003
Jasaszwili M, Wojciechowicz T, Billert M, Strowski MZ, Nowak KW, Skrzypski M (2019) Effects of adropin on proliferation and differentiation of 3T3-L1 cells and rat primary preadipocytes. Molecul cellular endocrinol 496:110532
Mitchell M, Armstrong DT, Robker RL, Norman RJ (2005) Adipokines: implications for female fertility and obesity. Reproduction 130(5):583–597
Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin MF, Murphy BD (2006) Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology 147(11):5178–5186
Acknowledgements
Shweta Maurya and Shashank Tripathi express sincere gratitude to CSIR, New Delhi, India, for providing fellowship as Junior and Senior Research Fellow. We also warmly thank the Department of Science and Technology-Funds for improvement of S & T infrastructure and the University Grant Commission-Centre of Advanced Study program to Department of Zoology, BHU, Varanasi, India. We are also most grateful to Mr. Sanjiv Singh, Department of Statistics, BHU, Varanasi, India for his statistical assistance.
Funding
This work was financially supported by DST-SERB (File no. ECR/2016/001883/LS), New Delhi, India.
Author information
Authors and Affiliations
Contributions
All authors conceptualized and designed the experiments. Shweta Maurya and Shashank Tripathi carried out all the experiments and prepared the figures and graphs. All authors analyzed the data. Shweta Maurya wrote the manuscript. Ajit Singh reviewed the work and edited the manuscript. All the authors read and agreed to publish the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Animal Ethical Committee (IAEC), Institute of Science, Banaras Hindu University authorized all experimental techniques, including animals (Mus musculus), in accordance with the ethical standards adopted by the Committee for the purpose of control and supervision of experiments on animals (CPCSEA), Government of India (BHU/ DoZ/ IAEC/2019-20/034).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maurya, S., Tripathi, S., Arora, T. et al. Adropin may regulate corpus luteum formation and its function in adult mouse ovary. Hormones 22, 725–739 (2023). https://doi.org/10.1007/s42000-023-00476-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-023-00476-0