Summary
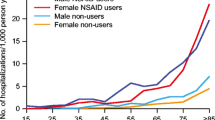
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most widely used drugs and their widespread use is associated with increased gastro-intestinal toxic effects such as ulceration, haemorrhage, perforation and death. They result in these complications mainly by reducing cytoprotective prostaglandins (PGE2 and PGI2) in the stomach, through the inhibition of cyclo-oxygenase (COX) enzyme. The increased morbidity and mortality, in addition to enormous cost, associated with NSAID-associated side effects, necessitates a need for safer GI-friendly NSAID.
Various approaches have been used to counteract NSAID associated side effects with varying degrees of success and acceptance. These include the use of alternative analgesia, anti-acid secretory agents like proton pump inhibitors, sucralfate and prostaglandin analogues. In addition, new types of NSAIDs are being developed, based on new understanding of their mechanism of action and the pathogenesis of inflammation. These include a new class of NSAIDs called “selective Cox-2 inhibitors”. These agents preserve the COX-1 that is responsible for the production of cytoprotective prostaglandins in the stomach and selectively inhibit COX-2 induced at the sites of inflammation. Selective COX-2 inhibitors exert the same analgesic and anti-inflammatory effects as the existing NSAIDs but may be less toxic to the stomach. In this review the background development and well-structured clinical trials on this new generation NSAIDs are discussed.
Similar content being viewed by others
References
Rheumatoid arthritis: A double-blind comparison with indomethacin and placebo. International Journal of Clinical Pharmacology 1976; 13: 292–7.
Juul-moller, S., Edvardsson, N., Jahnmatz, B. et al. Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. Lancet 1992; 340: 1421–1425.
ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither in 17187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2: 349–60.
Kirchheiner, B., Trang, L., Wellheim, F. A. Diclofenac sodium (Valtaren) in UK-TIA Study Group. United Kingdom transient ischaemic attack (UK-TIA) aspirin trial. J. Neurol. Neurosurg. Psychiatry 1991; 54: 1044–54.
Bousser, M. G., Eschwege, E., Haguenau, M, Lefauconnier, J. M., Thibult, N., Touboul, D. et al. “AICLA” controlled trial of aspirin and dipyridamole in the secondary prevention of athero-thrombotic cerebral ischemia. Stroke 1983; 14: 5–14.
Peleg, I., Maibach, H. T., Brown, S. H. et al. Aspirin and non-steroidal anti-inflammatory drug use and the risk of subsequent colorectal cancer. Arch. Intern. Med. 1994; 154: 394–399.
Waddell, W. R. Loughry, R. W. Sulindac for polyposis of the colon. J. Surg. Oncol. 1983; 24: 83–87.
Bateman, D. N. NSAlDs: Time to re-evaluate gut toxicity. Lancet 1994; 343: 1051–52.
Paulus, H.E. FDS Arthritis Advisory Committee meeting: Post-marketing surveillance of NSAIDs. Arthritis Rheum. 1985; 28: 1168–69.
Walt, R., Katschinski, B., Logan, R., Ashley, J., Langman, M. Rising frequency of ulcer perforation in elderly people in United Kingdom. Lancet 1986; 1: 489–92.
Somerville, K., Faulkner G., Langman, M. NSAID and bleeding peptic ulcer. Lancet 1986; 1: 462–66.
Bjarnason, I., Zanelli, G., Prowse, P. et al. Blood and protein loss via small intestinal inflammation induced by non steroidal anti-inflammatory drugs. Lancet 1987; 2: 711–714.
Matsuhashi, N., Yamada, A., Hiraishi, M., Konishi, S., Minota, S., Saito, T., Sugano, K., Yazaki, Y., Min, M., Shiga, J.Multiple strictures of small intestine after long term NSAID drug therapy. Am. J. Gastroenterol. 1992; 87: 3–86.
Slattery, J., Warlow, C. P., Shorrock, C. J. et al. Risk of gastro-intestinal bleeding during secondary prevention of vascular events with Aspirin. Analysis of gastro-intestinal bleeding during UK-T.I.A. trial. Gut 1995; 37: 509–511.
Weil, J., Colin-Jones, D., Langman, M. J. S. et al. Prophylactic Aspirin and the risk of peptic ulcer bleeding. BMJ 1995; 310: 827–30.
Rodriguez, L. A. G., Jick, H. Risk of upper gastrointestinal bleeding and perforation associated with individual NSAIDs. Lancet 1994; 343: 769–72.
Langman, M., Weil, J., Wainwright, P., Lawson, D. H., Rawlins, M. D., Logan, R. F. A., Murphy, M., Vessey, M. P., Colin-Jones, D. G. Risk of bleeding peptic ulcer associated with individual NSAIDs. Lancet 1994; 343: 1075–78.
Wallace, J. L., Tigley, A. W. New insights into prostaglandins and mucosal defence. Aliment. Pharmacol. Ther. 1995; 9: 227–235.
Ruppin, H., Person, B., Robert, A., Domschke, W. Gastric cytoprotection in man by Prostaglandin E2. Scand. J. Gastroenterol. 1981; 16: 647–52.
Kobayashi, K., Arakawa, T., Satoh, H., Fukuda, T., Nakamura, H. Effect of indomethacin, tiaprofenic acid diclofenac on rat gastric mucosal damage and content of prostacyclin and prostaglandin E2. Prostaglandins 1985; 30: 609–18.
Saroseik, J., Mizuta, K., Slomiany, A. et al. Effect of acetylsalicylic acid on gastric mucin viscosity, permeability to hydrogen ions, and susceptibility to pepsin. Biochem. Pharmacol. 1986; 35: 4291–95.
Fantone, J. C. Mechanism of chemotactic factor stimulation of polymorphonuclear leukocytes: modulation by prostaglandins. In: Higgs, G. A., Williams, T. J., eds. Inflammatory mediators. London: MacMillan 1985: 127–48.
Feldman, M., Colturi, T. J. Effect on indomethacin on gastric acid and bicarbonate secretions in humans. Gastroenterology 1984; 87: 1339–43.
Selling, J. A., Hogan, D. L., Aly, A. et al. Indomethacin inhibits duodenal mucosal bicarbonate secretion and endogenous prostaglandin E2 output in human subjects. Ann. Intern. Med. 1987; 106: 368–71.
Allison, M., Howatson, M., Torrance, C., Lee, F., Russell, R. Gastro-intestinal damage associated with the use of NSAIDs. N. Engl. J. Med. 1992; 327: 749–754.
Xie, W., Robertson, D. L., Simmons, D. L. Mitogen-inducible prostaglandin G/H synthase: a new target for NSAIDs. Drug Dev. Res. 1992; 25: 249–65.
Seibert, K., Zhang, Y., Leahy, K., Hauser, S. et al. Pharmacological and biochemical demonstration of the role of cyclo-oxygenase 2 in inflammation and pain. Proc. Natl. Acad. Sci. USA. 1994; 91: 12013–10217.
Vane, J. R., Mitchell, J. A., Appleton, I., Willoughby, D. A. et al. Inducible isoforms of cyclooxygenase and nitric oxide synthase in inflammation. Proc. Natl. Acad Sci. USA. 1994; 91:2046–2050.
Masferrer, J. L., Zweifel, B. S., Manning, P. T. et al. Selective inhibition of inducible cyclo-oxygenase 2 in vivo is anti-inflammatory and non ulcerogenic. Proc. Natl. Acad. Sci. USA. 1994; 91: 3228–3232.
Murray, F. E., Shah, A. A., Thjodleifsson, B., Oddsson, E., Gudjonsson, H., Sigthorsson, G., Crane, R., Bjarason, I., Fitzgerald, D. J. Comparison of the effects of naproxen and the COX-2 selective NSAID nimesulide, on prostanoid formation in man. Gut 1998; 42 (Suppl. 1); A6.
Glaser, K., Sung, M. L., O’Neill, K., Belfast, M. et al. Etodolac selectively inhibits human PGHS-1 versus PGHS- 2. Eur. J. Phamacol. 1995; 281: 107–111.
Laine, L., Sloane, R., Ferretti, M., Cominelli, F. A randomized double blind comparison of placebo etodolac, and naproxen on gastrointestinal injury and psotaglandin production; Gastrointestinal Endoscopy. 1995; 42: 428–33.
Cullun, L., Kelly, L., O’Conner, S., Fitzgerald, D. J. Nimesulide is selective COX-2 inhibitor in man. J. Pharmacol. Exp. Ther. 1998; 287: 578–582.
Marini, U., Spotti, D. Gastric tolerability of nimesulide. A double-blind comparison of two dosage regimens and placebo. Drugs 1993; 46S: 249–52
Shah, A. A., Murray, F. E., Thjodleifsson, B., Oddsson, E., Gudjonsson, H., Sigthorsson, G., Bjarason, I., Fitzgerald, D. J. Nimesulide, a selective COX-2 inhibitor, cause less gastro-intestinal damage compared with naproxen: A prospective study in human volunteers. Gut 1998; 42 (Suppl. 1); A68.
Bjarason, I., MacPherson, A., Rotman, H., Schupp, J., Hayllar, J. A randomized, double-blind, cross over, competitive endoscopy study on the gastro-intestinal tolerability of a highly specific COX-2 inhibitor flosulide and naproxen. Scand. J. Gastroenterol. 1997; 32: 126–30.
Bjarnason, I., Hayllar, J., MacPherson, Russell A. S. Side effects of NSAIDs on the small and large intestine in humans. Gastroenterology 1993; 104: 1832–47.
Meling.T. R., Aabakken, L., Roseth, A., Osnes, M. Feacal calprotectin shedding after short term treatment with NSAIDs. Scand. J. Gastroenterol. 1996; 31: 339–344.
Dreiser, R. L., Riebenfeld, D. Nimesulide in the treatment of osteoarthritis. Double blind studies in comparison with piroxicam, ketoprofen and placebo. Drug 1993; 46 (Suppl 1): 191–195.
Fossaluzza, V., Montagnani, G. Efficacy and tolerability of nimesulide in elderly patients with osteoarthritis: double blind trial versus naproxen. J. Int. Med. Res. 1989; 17: 295–303.
Mele, G., Memeo, A., Mellisi, L. et al. Post marketing surveillance on nimesulide in the treatment of 8354 patients over 60 years old affected with acute and chronic musculoskeletal diseases. Arch Med. Interna. (in Italian). 1992; 44: 213–21.
Pochobradsky, M. G., Mele, G. Bretta, A. Post marketing survey of nimesulide in the short term treatment of osteoarthritis. Drug Exper. Clin. Res. 1991; 17: 197–204.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shah, A.A., Fitzgerald, D.J. & Murray, F.E. Non-steroidal anti-inflammatory drugs (NSAIDs) and gastro-intestinal toxicity: Current issues. Ir. J. Med. Sc. 168, 242–245 (1999). https://doi.org/10.1007/BF02944348
Issue Date:
DOI: https://doi.org/10.1007/BF02944348