Abstract
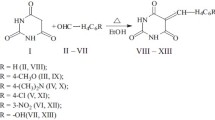
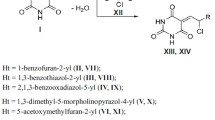
A series of 2,3-dihydroindole-2-thiones was evaluated forin vitro antimycobacterial activity againstMycobacterium tuberculosis, M. kansasii, M. fortuitum, two strains ofM. intracellulare and three strains ofM. avium. 2,3-Dihydro-1-methyl-2-thioxoindole-3-one and 2,3-dihydro-1-butyl-2-thioxoindole-3-one were the most active substances against potentially pathogenic strains, being more active than isoniazide.
Similar content being viewed by others
References
Heinisch L.:N-Substituted isatines from phenylnitrones of glyoxylic anilides.J.Prakt.Chem. 317, 435–447 (1975).
Šlosárek M., Lázničková T., Pellar T., Šeremek G.: Application of micromethods for testing drug sensitivity of mycobacteria.Stud.Pneumol.Phtiseol.Czechoslov. 49, 672–676 (1989).
Waisser K., Drhová L.: Advances in the development of new antituberculotics of the group of five-membered heterocyclic compounds containing one species of the heteroatom.Česká Slov.Farm. 48, 147–155 (1999).
Waisser K., Hladůvková J., Hrabálek A., Klimešová V., Kubicová L., Kuneš J., Macháček M., Palát K., Sova J., Vinšová J., Buchta V., Jilek P., Odlerova Z.: New pharmacophores of antimycobacterial and antimycotical activity, pp. 34–46 inPostepy Nauk Farmaceutycznych (J. Pachecka, J. Szewczynski, Eds). Polskie Towarzystwo Farmaceuticzne, Warszawa 1996.
Waisser K., Gregor J., Dostál H., Kuneš J., Kubicová L., Klimešová V., Kaustová J.: Influence of the replacement of the oxo function with the thioxo group an the antimycobacterial activity of 3-aryl-6,8-dichloro-2H-1,3-benzoxazine-2,4(3H)-diones and 3-arylquinazolinc-2,4(1H,3H)-diones.Farmaco 56, 803–807 (2001).
Waisser K., Dražková K., Čižmarik J., Kaustová J.: Antimycobacterial activity of basic ethyl esters of alkoxy-substituted phenylcarbamic acids.Folia Microbiol. 48, 45–50 (2003a).
Waisser K., Dražková K., Čižmárik J., Kaustová J.: Antimycobacterial activity of piperidinylpropyl esters of alkoxysubstituted phenylcarbamic acids.Folia Microbiol. 48, 585–587 (2003b).
Waisser K., Dražková K., Čižmárik J., Kaustová J.: Influence of lipophilicity on the antimycobacterial activity of the hydrochlorides of piperidinylethyl esters ofortho-substituted phenylcarbamic acids.Sci.Pharm. 72, 43–49 (2004a).
Waisser K., Dražková K., Čižmárik J., Kaustová J.: New group of potential antituberculotics: hydrochlorides of piperidinylalkyl esters of alkoxysubstituted phenylcarbamic acid.Folia Microbiol. 49, 265–268 (2004b).
Waisser K., Adamec J., Doležal R., Kaustová J.: 1-Aryl-5-benzylsulfanyltetrazoles, a new group of antimycobacterial compounds against potentially pathogenic strains.Folia Microbiol. 50, 195–197 (2005a).
Waisser K., Heinisch L., Šlosárek M., Janota J.: New antimycobacterialS-alkylisothiosemicarbazones.Folia Microbiol. 50, 479–481 (2005b).
Author information
Authors and Affiliations
Additional information
The work was a part of the research project no. MSM 0021 620 822 of theMinistry of Education, Youth and Sports of the Czech Republic.
Rights and permissions
About this article
Cite this article
Waisser, K., Heinisch, L., Šlosárek, M. et al. New antimycobacterial 2,3-dihydro-1-alkylindole-2-thiones. Folia Microbiol 51, 25–26 (2006). https://doi.org/10.1007/BF02931445
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02931445