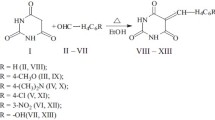
Series of 5-(hetarylmethylidene)-2,4,6-pyrimidine-2,4,6(1H,3H,5H)-triones and 5-(2-chloropropylidene)- 2,4,6-pyrimidine-2,4,6(1H,3H,5H)-triones were synthesized and tested for antimycobacterial activity against M. lufu and acute toxicity. The 5-(hetarylmethylidene)-2,4,6-pyrimidine-2,4,6(1H,3H,5H)-triones and 5-(2-chloropropylidene)-2,4,6-pyrimidine-2,4,6(1H,3H,5H)-triones were shown to possess low toxicity and antimycobacterial activity of various strengths as compared to dapsone and could be considered promising compounds for further discovery of antimycobacterial drugs.
Similar content being viewed by others
References
Chemotherapy of Leprosy for Control Programmes, WHO Tech. Rep. Ser. 675, Geneva (1982).
S. K. Noordeen, Clin. Dermatol., 34, 32 – 36 (2016).
A. A. Kubanov, A. E. Karamova, A. A. Vorontsova, and P. A. Kalinina, Vestn. Dermatol. Venerol., 4, 12 – 19 (2016).
H. K. Kar and R. Gupta, Clin. Dermatol., 33, 55 – 56 (2015).
V. Z. Naumov, E. A. Zadneprovskaya, and A. A. Yushchenko, Vestn. Dermatol. Venerol., 6, 21 – 23 (2006).
A. R. Gimadieva, Yu. N. Chernyshenko, A. G. Mustafin, and I. B. Abdrakhmanov, Bashk. Khim. Zh., 14(3), 5 – 21 (2007).
A. G. Tyrkov, N. G. Urlyapova, and A. D. Daudova, Khim.- farm. Zh., 40(7), 30 – 31 (2006); Pharm. Chem. J., 40(7), 377 – 379 (2006).
S. A. Luzhnova, A. G. Tyrkov, N. M. Gabitova, and E. A. Yurgaeva, Khim.-farm. Zh., 49(12), 12 – 14 (2015); Pharm. Chem. J., 49(12), 810 – 812 (2016).
E. A. Yurgaeva and A. G. Tyrkov, Zh. Org. Khim., 52(2), 305 – 307 (2016); Russ. J. Org. Chem., 52(2), 289 – 291 (2016).
E. Pretsch, P. Buhlmann, and C. Affolter, Structure Determination of Organic Compounds, Springer, Berlin, New York (2000) [Russian translation, Binom, Laboratoriya Znanii, Mir, Moscow (2006), pp. 393 – 395].
A. A. Potekhin, Properties of Organic Compounds [in Russian], Khimiya, Leningrad (1984), p. 296.
J. G. Kirchner, Techniques of Chemistry, Vol. 14: Thin-Layer Chromatography, 2nd Ed., Wiley-Interscience, New York (1978), 1137 pp [Russian translation, Vol. 1, Mir, Moscow (1981), pp. 129, 218].
F. Portaels, Ann. Soc. Belge Med. Trop., 60, 381 – 386 (1980).
J. K. Seydel and E. C. Wempe, Int. J. Lepr., 50(1), 20 – 30 (1982).
O. A. Irtuganova and N. G. Urlyapova, Aktual. Vopr. Lepr. (Astrakhan’), 148 – 150 (1984).
F. Portaels, XIIth International Leprosy Congress: Abstracts, New Delhi, IV, 220 A (1984).
S. M. Navashin and I. P. Fomina, Handbook of Antibiotics [in Russian], Meditsina, Moscow (1974), p. 54.
Handbook for Preclinical Drug Trials [in Russian], Grif i K, Moscow (2012), p. 944.
S. Glantz, Primer of Biostatistics, 4th Ed., McGraw-Hill Inc., New York (1997), 473 pp [Russian translation, Praktika, Moscow (1999), p. 459].
M. D. Mashkovskii, Drugs [in Russian], Novaya Volna, Moscow (2006), p. 873.
A. G. Pashinyan, Med. Sovet, 3, 4, 46 – 47 (2011).
N. Coen, S. Duraffour, D. Topalis, R. Snoeck, and G. Andrei, Antimicrob. Agents Chemother., 58(12), 7312 – 7323 (2014).
Acknowledgments
The work was performed in the framework of a State Task for the Ministry of Health of Russia, Project No. 056-00074-19-00 and was financially supported by the Ministry of Education and Science of the RF, Grant No. 4.9288.2017BCh.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 54, No. 2, pp. 32 – 35, February, 2020.
Rights and permissions
About this article
Cite this article
Yushin, M.Y., Tyrkov, A.G., Saroyants, L.V. et al. Synthesis and Antimycobacterial Activity of 5-(Hetarylmethylidene)-2,4,6-Pyrimidine- 2,4,6(1H,3H,5H )-Triones And 5-(2-Chloropropylidene)- 2,4,6-Pyrimidine-2,4,6(1H,3H,5H )-Triones. Pharm Chem J 54, 134–137 (2020). https://doi.org/10.1007/s11094-020-02169-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02169-z