Abstract
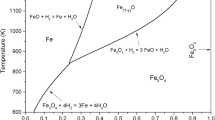
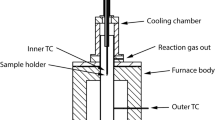
α-Hematite powder having an average diam of 8.1 μm was packed in a vertical quartz reactor (I.D. 14.6 mm) and was reduced by feeding hydrogen toward the top of the packed bed at 900°C under atmospheric pressure. A large increase in pressure as high as 118 mmHg was observed within the powder bed during the course of reaction. The stoichiometric condition that the diffusion rate of the reactant gas, H2, should be equal to that of the product gas, H2O, gave rise to this pressure increase within the bed. When the pressure within the bed were uniform, the ratio of the diffusional fluxes of the reactant gas, H2, and of the product gas, H2O, would be equal to the square root of the ratio of the molecular weight of H2O and of H2. An effective diffusivity which includes not only the diffusional term but also the hydrodynamic term was introduced into the equations for the flow of gases with chemical reactions. The pressure variations within the powder bed calculated from the proposed flow equations agreed quite well with those observed during the course of the three-step reduction of hemattte (Fe2O3) to magnetite (Fe3O4), then to wustite (Fe1−y O), and finally to iron. It was also found that, during the reduction, the presence of pressure gradient within the bed reduced the effective diffusivity of hydrogen to about one third of that without the pressure gradient.
Similar content being viewed by others
References
R. G. Olsson and W. M. McKewan:Met. Trans., 1970, vol. 1, pp. 1507–12.
E. T. Turkdogan and J. V. Vinters:Met. Trans., 1971, vol. 2, pp. 3175–88; 1971, vol. 2, pp. 3189–96; 1972, vol. 3, pp. 1561–74; 1972, vol. 3, pp. 2039–48.
R. G. Olsson and W. M. McKewan:Trans. TMS-AIME, 1966, vol. 236, pp. 1518–22.
R. B. Evans, III, G. M. Watson, and E. A. Mason:J. Chem. Phys., 1961, vol. 35, pp. 2076–83.
E. A. Mason, A. P. Malinauskas, and R. B. Evans, III:J. Chem. Phys., 1967. vol. 46, pp. 3199–3219.
N. Wakao, S. Otani, and J. M. Smith:A.I.Ch.E. J., 1965, vol. 11, pp. 435–39.
R. D. Gunn and C. J. King:A.I.Ch.E. J., 1969, vol. 15, pp. 507–14.
Y. Nakano: M.S. Thesis, Tokyo Inst. Tech., 1970.
L. Himmel, R. F. Mehl, and C. F. Birchenall:Trans. AIME, 1953, vol. 197. pp. 827–43 (Journal of Metals, June-1953).
E. Wicke and R. Kallenbach:Kolloid Z., 1941, vol. 97, pp. 135–51.
R. H. Spitzer, F. S. Manning, and W. O. Phibrook:Trans. TMS-AIME, 1966, vol. 236, pp. 1715–24.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakano, Y., Ishida, M., Akehata, T. et al. Pressure increase and “dynamic effective diffusivity” within a powder bed during reaction-reduction of iron oxide powders by hydrogen at 900°C. Metall Trans B 6, 429–434 (1975). https://doi.org/10.1007/BF02913828
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02913828