Abstract
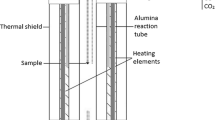
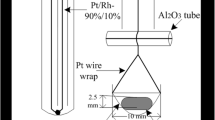
The distribution of silver between molten metal and matte was determined at temperatures of 1150 to 1250°C. The partition coefficient (KAg-ratio of wt pct silver in matte to wt pct silver in metal) was 0.46 and was essentially independent of silver concentration, temperature, iron content and oxygen and SO2 partial pressures over the ranges studied. Only the presence of nickel significantly affected KAg, causing it to rise from 0.46 to 1.16 within the region of liquid immiscibility. Outside this region all silver was intimately associated with chalcocite, rather than the heazel woodite or metallics, formed during solidification. Studies on other precious metals showed that KAu=8×10−3, KPd=6×10−3 and KPt=4×10−4 in the Cu−S system at 1200°C. Application of these results to actual operations is discussed, as is the chemical behavior of precious metals during matte smelting.
Similar content being viewed by others
References
N. Asano:Nippon Kogyo Kaishi, 1965, vol. 81, pp. 283–85.
N. Asano and M. Wada:Nippon Kogyo Kaishi, 1963, vol. 79, pp. 235–36.
N. Asano and T. Ichio:Suiyokwaishi, 1962, vol. 14, pp. 467–70.
N. Asano, K. Waso, and M. Kobayoshi,Nippon Kogyo Kaishi, 1971, vol. 87, pp. 347–52.
W. J. Schlitt and K. J. Richards:Met. Trans., 1973, vol. 4, pp. 819–25.
W. A. Baker and A. P. C. Hallowes:Trans. Inst. Mining Met. (London), 1949–1950, vol. 59, pp. 129–46.
W. J. Schlitt, R. H. Craig, and K. J. Richards:Met. Trans., 1973, vol. 4, pp. 1994–96.
R. W. Ruddle:The Physical Chemistry of Copper Smelting, p. 16, Inst. Mining Met. (London), 1953.
J. Gerlach, U. Hennig, and K. Trettin:Z. Erzbergbau Metallhuettenw., 1966, vol. 19, pp. 458–63.
H. Diettrick, J. Gerlach, and U. Hennig:Z. Erzbergbau Metallhuettenw., 1967, vol. 20, pp. 308–14.
J. Gerlach, U. Hennig, and K. Trettin:Erzmetall., 1969, vol. 22, pp. 119–22.
J. Gerlach, U. Hennig, and H.-S. Park:Erzmetall., 1972, vol. 25, pp. 69–77.
E. A. Peretti: inCopper, pp. 149–64, Hafner Publishing Co., 1970.
J. R. Boldt, Jr.:The Winning of Nickel p. 278, D. Van Nostrand Co., Inc., 1967.
K. Sproule, G. A. Harcourt, and L. S. Renzoni: inExtructive Metallurgy of Copper, Nickel and Cobalt, pp. 33–54, Interscience Publishers, 1961.
R. Hultgren,et al.: Selected Values of the Thermodynamic Properties of Binary Alloys, pp. 48, 268, 780, 785, ASM, 1973.
M. Hansen and K. Anderko:Constitution of Binary Alloys, 2nd ed., pp. 18, 36, 199, 613, 617, McGraw-Hill Book Co., 1958.
E. G. King, A. D Mah, and L. B. Pank:atz:Thermochemical Properties of Copper and Its Inorganic Compounds, (INCRA Monograph II), p. 90, INCRA and U.S. Bur. Mines, 1973.
O. Kubaschewski, E Li. Evans, and C. B. Alcock:Metallurgical Thermochemistry, 4th ed., p. 421, Pergamon Press, 1967.
I. Barin and O. Knacke:Thermochemical Properties of Inorganic Substances, p. 6, Springer-Verlag, 1973.
H. H. Kellogg:Can. Met. Quart., 1969, vol. 8, pp. 3–23.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schlitt, W.J., Richards, K.J. The distribution of silver, gold, platinum and palladium in metal-matte systems. Metall Trans B 6, 237–243 (1975). https://doi.org/10.1007/BF02913565
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02913565