Summary
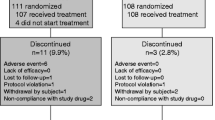
To evaluate the efficacy and safety of risedronate sodium in treatment of postmenopausal osteoporosis, one-year randomized, double blind clinical trial was performed among 54 women with postmenopausal osteoporosis. The changes were compared in bone mineral density (BMD), bone metabolism markers and adverse events after 12 months oral administration of risedronate sodium. BMD was measured by dual energy X-ray absorptionmetry (DEXA) and bone turnover marker was detected. The results showed that there was a significant increase in BMD of the lumbar spine (3.29%±1.18%, 4.51%±1.64% respectively) after 6 and 12 months in the risedronate treatment group versus placebo control group (−0.62%±0.24%, 0.48%±0.18% respectively). Bone turnover was decreased to a stable nadir over 6 and 12 months for resorption markers [N-telopeptide (NTx),P<0.05] and over 12 months for formation marker (ALP,P<0.05; BGP,P<0.05). The safety profile of risedronate sodium was similar to that of placebo. There were no trends toward increased frequency of any adverse experience except for gastrointestinal symptoms (7.1%), rash (7.1%) and hematuria (3.6%), which were usually mild, transient, and resolved with continued treatment. It was concluded that risedronate was an efficacious and safe drug in treatment of postmenopausal osteoporosis.
Similar content being viewed by others
References
Borah B, Dufresne T E, Chmielewski P Aet al. Risedronate preserves bone architecture in postmenopausal women with osteoporosis as measured by three-dimensional microcomputed tomography. Bone, 2004, 34(4): 736
Harrington J T, Ste-Marie L G, Brandi M Let al. Risedronate rapidly reduces the risk for nonvertebral fractures in women with postmenopausal osteoporosis. Calcif Tissue Int, 2004, 74(2): 129
Fogelman I, Ribot C, Smith Ret al. Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. J Clin Endocrinol Metab, 2000, 85: 1895
Rozkydal Z, Janicek P. The effect of alendronate in the treatment of postmenopausal osteoporosis. Bratisl Lek Listy, 2003, 104(10): 309
Lufkin E G, Sarkar S, Kulkarni P Met al. Antiresorptive treatment of postmenopausal osteoporosis: review of randomized clinical studies and rationale for the Evista alendronate comparison (EVA) trial. Curr Med Res Opin, 2004, 20(3): 351
Kimmel D B, Jee W S. A quantitative histologic study of bone turnover in young adult beagles. Anat Rec, 1982, 203: 31
Gatti D, Adami S. New bisphosphonates in the treatment of bone disease. Drugs Aging, 1999, 15: 285
Giuliani N, Pedrazzoni M, Passeri Get al. Bisphosphonates inhibit IL-6 production by human osteoblastliks cells. Scand J Rheumatol, 1998, 27: 38
Fleisch H. Bisphosphonates: mechanisms of action. Endo Revi, 1998, 19: 80
Luckman S P, Hughs D E, Coxon F Pet al. Netrogencontaining bisphosphonates inhabit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including ras. J Bone Miner Res, 1998, 13: 1668
Ross P D, Kress B C, Parson R Eet al. Serum bone alkaline phosphatase and calcaneus bone density predict factors: A prospective study. Osteopors Int, 2000, 11 (1): 76
Dresner-pollak R, Karmeli F, Eliakin Ret al. Increased urinary N-telopetide cross-linked type I collagen predicts bone loss in patients with inflammatory bowel disease. Am J Gastroenterol, 2000, 95(3): 699
Hamilton B, McCoy K, Taggart H. Tolerability and compliance with risedronate in clinical practice. Osteoporos Int, 2003, 14(3): 259
Author information
Authors and Affiliations
Additional information
LI Yuming, male, born in 1962, Associate Professor
Rights and permissions
About this article
Cite this article
Yuming, L., Zhongzhi, Z., Xiuling, D. et al. Efficacy and safety of risedronate sodium in treatment of postmenopausal osteoporosis. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 25, 527–529 (2005). https://doi.org/10.1007/BF02896007
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02896007