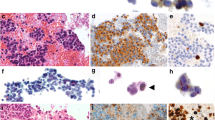
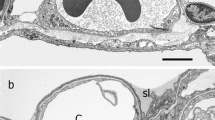
Summary
The various epithelial cells of the lower respiratory tract and the carcinomas derived from them differ markedly in their differentiation characteristics. Using immunofluorescence microscopy and two-dimensional gel electrophoresis of cytoskeletal proteins from microdissected tissues we have considered whether cytokeratin polypeptides can serve as markers of cell differentiation in epithelia from various parts of the human and bovine lower respiratory tract. In addition, we have compared these protein patterns with those found in the two commonest types of human lung carcinoma and in several cultured lung carcinoma cell lines. By immunofluorescence microscopy, broad spectrum antibodies to cytokeratins stain all epithelial cells of the respiratory tract, including basal, ciliated, goblet, and alveolar cells as well as all tumor cells of adenocarcinomas and squamous cell carcinomas. However, in contrast, selective cytokeratin antibodies reveal cell type-related differences. Basal cells of the bronchial epithelium react with antibodies raised against a specific epidermal keratin polypeptide but not with antibodies derived from cytokeratins characteristic of simple epithelia. When examined by two-dimensional gel electrophoresis, the alveolar cells of human lung show cytokeratin polypeptides typical of simple epithelia (nos. 7, 8, 18 and 19) whereas the bronchial epithelium expresses, in addition, basic cytokeratins (no. 5, small amounts of no. 6) as well as the acidic polypeptides nos. 15 and 17. Bovine alveolar cells also differ from cells of the tracheal epithelium by the absence of a basic cytokeratin polypeptide. All adenocarcinomas of the lung reveal a “simple-epithelium-type” cytokeratin pattern (nos. 7, 8, 18 and 19). In contrast, squamous cell carcinomas of the lung contain an unusual complexity of cytokeratins. We have consistently found polypeptides nos. 5, 6, 8, 13, 17, 18 and 19 and, in some cases, variable amounts of cytokeratins nos. 4, 14 and 15.
Several established cell lines derived from human lung carcinomas (SK-LU-1, Calu-1, SK-MES-1 and A-549) show a uniform pattern of cytokeratin polypeptides (nos. 7, 8, 18 and 19), similar to that found in adenocarcinomas. In addition, vimentin filaments are produced in all the cell lines examined, except for SK-LU-1.
From these analyses we conclude, in contrast to some previous reports, (1) that all epithelial cells from the trachea and lung contain cytokeratins, (2) that epithelial cells from different parts of the respiratory tract express different cytokeratins, and (3) that the bronchial epithelium is characterized by the occurrence of basic (nos. 5 and 6) and acidic (nos. 15 and 17) polypeptides which are absent from alveoli. A marked, probably related difference in cytokeratin expression is also seen between squamous cell carcinomas and adenocarcinomas.
The possible value of analyses of cytokeratin polypeptide patterns in lung carcinomas and metastases is discussed in relation to the diagnosis and histogenesis of lung neoplasms. We propose to use monoclonal antibodies specific for individual cytokeratin polypeptides as selective diagnostic tools for the differentiation and classification of lung carcinomas.
Similar content being viewed by others
References
Altmannsberger M, Osborn M, Schauer A, Weber K (1981) Antibodies to different intermediate filament proteins. Cell type-specific markers on paraffin-embedded human tissues. Lab Invest 45:427–434
Ansorge W (1983) Fast visualization of protein bands by impregnation in potassium permanganate and silver nitrate. In: Stathakos D (ed) Proceedings of the 3rd international conference on electrophoresis. De Gruyter, Berlin
Bannasch P, Zerban H, Schmid E, Franke WW (1980) Liver tumors distinguished by immunofluorescence microscopy with antibodies to proteins of intermediate-sized filaments. Proc Natl Acad Sci USA 77:4948–4952
Bejui-Thivolet F, Viac J, Thivolet J, Faure M (1982) Intracellular keratins in normal and pathological bronchial mucosa. Virchows Arch (Pathol Anat) 395:87–98
Bernai SD, Baylin SB, Shaper JH, Gazdar AF, Chen LB (1983) Cytoskeleton-associated proteins of human lung cancer cells. Cancer Res 43:1798–1808
Caselitz J, Osborn M, Scifert G, Weber K (1981) Intermediate-sized filament proteins (prekeratin, vimentin, desmin) in the normal parotid gland and parotid gland tumours. Virchows Arch [Pathol Anat] 393:273–286
Debus E, Weber K, Osborn M (1982) Monoclonal cytokeratin antibodies that distinguish simple from stratified squamous epithelia: characterization on human tissues. EMBO J 1:1641–1647
Debus E, Moll R, Franke WW, Weber K, Osborn M (1984) Immunohistochemical distinction of human carcinomas by cytokeratin typing with monoclonal antibodies. Am J Pathol 114:121–130
Denk H, Krepier R, Lackinger E, Artlieb U, Franke WW (1982) Biochemical and immunocytochemical analysis of the intermediate filament cytoskeleton in human hepatocellular carcinomas and in hepatic nodules of mice. Lab Invest 46:584–596
Fogh J, Trempe G (1975) New human tumor cell lines. In: Fogh J (ed) Human tumor cells in vitro. Plenum Press, New York, London, pp 115–159
Fogh J, Fogh JM, Orfeo T (1977a) One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst 59:221–226
Fogh J, Wright WC, Loveless JD (1977b) Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J Natl Cancer Inst 58:209–214
Franke WW, Schmid E, Osborn M, Weber K (1978a) Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci USA 75:5034–5038
Franke WW, Weber K, Osborn M, Schmid E, Freudenstein C (1978b) Antibody to prekeratin. Decoration of tonofilament-like arrays in various cells of epithelial character. Exp Cell Res 116:429–445
Franke WW, Appelhans B, Schmid E, Freudenstein C, Osborn M, Weber K (1979a) Identification and characterization of epithelial cells in mammalian tissues by immunofluorescence microscopy using antibodies to prekeratin. Differentiation 15:7–25
Franke WW, Schmid E, Breitkreutz D, Lüder M, Boukamp P, Fusenig NE, Osborn M, Weber K (1979b) Simultaneous expression of two different types of intermediate-sized filaments in mouse keratinocytes proliferating in vitro. Differentiation 14:35–50
Franke WW, Schmid E, Kartenbeck J, Mayer D, Hacker HJ, Bannasch P, Osborn M, Weber K, Denk H, Wanson J-C, Drochmans P (1979c) Characterization of the intermediate-sized filaments in liver cells by immunofluorescence and electron microscopy. Biol Cell 34:99–110
Franke WW, Schmid E, Weber K, Osborn M (1979d) HeLa cells contain intermediatesized filaments of the prekeratin type. Exp Cell Res 118:95–109
Franke WW, Schmid E, Winter S, Osborn M, Weber K (1979e) Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res 123:25–46
Franke WW, Schmid E, Freudenstein C, Appelhans B, Osborn M, Weber K, Keenan TW (1980) Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol 84:633–654
Franke WW, Mayer D, Schmid E, Denk H, Borenfreund E (1981a) Differences of expression of cytoskeletal proteins in cultured rat hepatocytes and hepatoma cells. Exp Cell Res 134:345–365
Franke WW, Schiller DL, Moll R, Winter S, Schmid E, Engelbrecht I, Denk H, Krepier R, Platzer B (1981b) Diversity of cytokeratins: differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol 153:933–959
Franke WW, Winter S, Grund C, Schmid E, Schiller DL, Jarasch E-D (1981c) Isolation and characterization of desmosome-associated tonofilaments from rat intestinal brush border. J Cell Biol 90:116–127
Franke WW, Schmid E, Schiller DL, Winter S, Jarasch E-D, Moll R, Denk H, Jackson B, Illmensee K (1982) Differentiation-related patterns of expression of proteins of intermediate-sized filaments in tissues and cultured cells. Cold Spring Harbor Symp Quant Biol 46:431–453
Franke WW, Moll R, Mueller H, Schmid E, Kuhn C, Krepier R, Artlieb U, Denk H (1983) Immunocytochemical identification of epithelium-derived human tumors using antibodies to desmosomal plaque proteins. Proc Natl Acad Sci USA 80:543–547
Fuchs E, Green H (1981) Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell 25:617–625
Fuchs EV, Coppock SM, Green H, Cleveland DW (1981) Two distinct classes of keratin genes and their evolutionary significance. Cell 27:75–84
Gabbiani G, Kapanci Y, Barrazone P, Franke WW (1981) Immunochemical identification of intermediate-sized filaments in human neoplastic cells: a diagnostic aid for the surgical pathologist. Am J Pathol 104:206–216
Gatter KC, Abdulaziz Z, Beverley P, Corvalan JRF, Ford C, Lane EB, Mota M, Nash JRG, Pulford K, Stein H, Taylor-Papadimitriou J, Woodhouse C, Mason DY (1982) Use of monoclonal antibodies for the histopathological diagnosis of human malignancy. J Clin Pathol 35:1253–1267
Giard DJ, Aaronson SA, Todarao GJ, Arnstein P, Kersey JH, Dosik H, Parks WP (1973) In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst 51:1417–1423
Gigi O, Geiger B, Eshhar Z, Moll R, Schmid E, Winter S, Schiller DL, Franke WW (1982) Detection of a cytokeratin determinant common to diverse epithelial cells by a broadly cross-reacting monoclonal antibody. EMBO J 1:1429–1437
Gould VE (1983) The endocrine lung. Lab Invest 48:507–509
Gould VE, Memoli VA, Dardi LE (1981) Multidirectional differentiation in human epithelial cancers. J Submicroscop Cytol 13:97–115
Gould VE, Linnoila RI, Memoli VA, Warren WH (1983) Neuroendocrine cells and neuroendocrine neoplasms of the lung. In: Sommers SC, Rosen PP (eds) Pathology Annual, Part I, vol 18. Appleton-Century-Crofts, Norwalk, Connecticut, pp 287–330
Gown AM, Vogel AM (1982) Monoclonal antibodies to intermediate filament proteins of human cells: unique and cross-reacting antibodies. J Cell Biol 95:414–424
Greenberg SD (1982) Histopathology and ultrastructure of bronchiolo-alveolar carcinoma. In: Shimosata Y, Nettesheim P, Melamed MR (eds) Morphogenesis of lung cancer, vol I. CRC Press Inc, Boca Raton, Florida, pp 121–145
Gusterson B, Mitchell D, Warburton M, Sloane J (1982) Immunohistochemical localisation of keratin in human lung tumours. Virchows Arch [Pathol Anat] 394:269–277
Kahn HJ, Hanna W, Yeger H, Baumal R (1982) Imunohistochemical localization of prekeratin filaments in benign and malignant cells in effusions. Am J Pathol 109:206–214
Kameya T, Shimosato Y, Kodama T, Tsumuraya M, Koide T, Yamaguchi K, Abe K (1983) Peptide hormone production by adenocarcinomas of the lung; its morphologic basis and histogenetic considerations. Virchows Arch [Pathol Anat] 400:245–257
Krepier R, Denk H, Artlieb U, Moll R (1982) Immunocytochemistry of intermediate filament proteins present in pleomorphic adenomas of the human parotid gland: characterization of different cell types in the same tumor. Differentiation 21:191–199
Lane EB (1982) Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilaments organization. J Cell Biol 92:665–673
Lane EB, Hogan BLM, Kurkinen M, Garrels JI (1983) Coexpression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo. Nature 303:701–704
Lehto V-P, Stenman S, Miettinen M, Dahl D, Virtanen I (1983) Expression of a neural type of intermediate filament as a distinguishing feature between oat cell carcinoma and other lung cancers. Am J Pathol 110:113–118
Lehtonen E, Lehto V-P, Paasivuo R, Virtanen I (1983) Parietal and visceral endoderm differ in their expression of intermediate filaments. EMBO J 2:1023–1028
McDowell EM, Harris CC, Trump BF (1982) Histogenesis and morphogenesis of bronchial neoplasms. In: Shimosato Y, Nettesheim P, Melamed MR (eds) Morphogenesis of lung cancer, vol I. CRC Press Inc, Boca Raton, Florida, pp 1–36
Mitchell DP, Gusterson BA (1982) Simultaneous demonstration of keratin and mucin. J Histochem Cytochem 30:707–709
Moll R, von Bassewitz DB, Schulz U, Franke WW (1982a) An unusual type of cytokeratin filament in cells of a human cloacogenic carcinoma derived from the anorectal transition zone. Differentiation 22:25–40
Moll R, Franke WW, Schiller DL, Geiger B, Krepier R (1982b) The catalog of human cytokeratin polypeptides: patterns of expression of specific cytokeratins in normal epithelia, tumors and cultured cells. Cell 31:11–24
Moll R, Franke WW, Volc-Platzer B, Krepier R (1982c) Different keratin polypeptides in epidermis and other epithelia of human skin: a specific cytokeratin of molecular weight 46,000 in epithelia of the pilosebaceous tract and basal cell epitheliomas. J Cell Biol 95:285–295
Moll R, Krepier R, Franke WW (1983a) Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation 23:256–269
Moll R, Levy R, Czernobilsky B, Hohlweg-Majert P, Dallenbach-Hellweg G, Franke WW (1983b) Cytokeratins of normal epithelia and some neoplasms of the female genital tract. Lab Invest 49:599–610
Nagle RB, McDaniel KM, Clark VA, Payne CM (1983) The use of antikeratin antibodies in the diagnosis of human neoplasms. Am J Clin Pathol 79:458–466
O’Farrell PH (1975) High resolution of two-dimensional electrophoresis of proteins. J Biol Chem 250:4007–4021
O’Farrell PZ, Goodman HM, O’Farrell PH (1977) High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell 12:1133–1142
Osborn M, Weber K (1983) Biology of disease. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest 48:372–394
Osborn M, Franke WW, Weber K (1980) Direct demonstration of the presence of two immunologically distinct intermediate sized filament systems in the same cell by double immunofluorescence microscopy. Exp Cell Res 125:37–46
Ramaekers FCS, Haag D, Kant A, Moesker O, Jap PHK, Vooijs GP (1983a) Co-expression of keratin- and vimentin-type intermediate filaments in human metastatic carcinoma cells. Proc Natl Acad Sci USA 80:2618–2622
Ramaekers FCS, Puts JJG, Moesker O, Kant A, Huysmans A, Haag D, Jap PHK, Herman CJ, Vooijs GP (1983b) Antibodies to intermediate filament proteins in the immunohisto-chemical identification of human tumours: an overview. Histochem J 15:691–713
Saba SR, Espinoza CG, Richman AV, Azar HA (1983) Carcinomas of the lung: an ultrastructural and immunocytochemical study. Am J Clin Pathol 80:6–13
Said JW, Nash G, Tepper G, Banks-Schlegel S (1983) Keratin proteins and carcinoembryonic antigen in lung carcinoma: an immunoperoxidase study of fifty-four cases, with ultrastructural correlations. Human Pathol 14:70–76
Schiller DL, Franke WW, Geiger B (1982) A subfamily of relatively large and basic cytokeratin polypeptides as defined by peptide mapping is represented by one or several polypeptides in epithelial cells. EMBO J 1:761–769
Schlegel R, Banks-Schlegel S, McLeod JA, Pinkus GS (1980a) Immunoperoxidase localization of keratin in human neoplasms. Am J Pathol 110:41–49
Schlegel R, Banks-Schlegel S, Pinkus GS (1980b) Immunohistochemical localization of keratin in normal human tissues. Lab Invest 42:91–96
Sherwin RP, Richters A (1975) Human lung cancer in tissue culture. In: Fogh J (ed) Human tumor cells in vitro. Plenum Press, New York, London, pp 267–297
Sieinski W, Dorsett B, Ioachim HL (1981) Identification of prekeratin by immunofluorescence staining in the differential diagnosis of tumors. Human Pathol 12:452–458
Sorokin SP (1977) The respiratory system. In: Weiss L, Greep RO (eds) Histology. McGraw-Hill Book Company, New York, pp 765–830
Sun TT, Green H (1978) Immunofluorescent staining of keratin fibers in cultured cells. Cell 14:469–476
Sun T-T, Shih CH, Green H (1979) Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci USA 76:2813–2817
Tseng SCG, Jarvinen MJ, Nelson WG, Huang J-W, Woodcock-Mitchell J, Sun T-T (1982) Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell 30:361–372
World Health Organization, Geneva (1982) The world health organization histological typing of lung tumors. Second Edition Am J Clin Pathol 77:123–136
Wu Y-J, Rheinwald JG (1981) A new small (40 kd) keratin filament protein made by some cultured human squamous cell carcinomas. Cell 25:627–635
Wu Y-J, Parker LM, Binder NE, Beckett MA, Sinard JH, Griffiths CT, Rheinwald JG (1982) The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell 31:693–703
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blobel, G.A., Moll, R., Franke, W.W. et al. Cytokeratins in normal lung and lung carcinomas. Virchows Archiv B Cell Pathol 45, 407–429 (1984). https://doi.org/10.1007/BF02889883
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02889883